
A��B��C��D��E��F����6��Ԫ�ء�����գ�
(1)AԪ�ػ�̬ԭ�ӵ��������2��δ�ɶԵ��ӣ��������2�����ӣ���Ԫ�ط���Ϊ__________��
(2)BԪ�صĸ�һ�����Ӻ�CԪ�ص���һ�����ӵĵ��Ӳ�ṹ�������ͬ��B��Ԫ�ط���Ϊ________��C��Ԫ�ط���Ϊ________��
(3)DԪ�ص����������ӵ�3d���Ϊ�������D��Ԫ�ط���Ϊ________�����̬ԭ�ӵĵ����Ų�ʽΪ_________________________________________________________________��
(4)EԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ�E��Ԫ�ط���Ϊ________�����̬ԭ�ӵĵ����Ų�ʽΪ__________________________________��
(5)FԪ�ص�ԭ�����������Ų�ʽΪnsnnpn��1����n��________��ԭ����������ߵ���________���ӡ�
�𰸡�(1)C��O��(2)Cl��K
(3)Fe��1s22s22p63s23p63d64s2��[Ar]3d64s2
(4)Cu��1s22s22p63s23p63d104s1��[Ar]3d104s1
(5)2��2p
������(1)AԪ�ػ�̬ԭ�Ӵ������2�����ӣ��ʴ����ΪK�㣬AԪ����2�����Ӳ㣬�������д��������ʾʽΪ ���Ԫ�غ�����6�����ӣ�Ϊ̼Ԫ�أ���Ԫ�ط���ΪC��������ԭ��ͬ��Ҳ����Ҫ��������ʾʽΪ
���Ԫ�غ�����6�����ӣ�Ϊ̼Ԫ�أ���Ԫ�ط���ΪC��������ԭ��ͬ��Ҳ����Ҫ��������ʾʽΪ
 ��
��
(2)B����C���ĵ��Ӳ�ṹ����Ar��ͬ�������ⶼ��18�����ӣ���BΪ17��Ԫ��Cl��CΪ19��Ԫ��K��
(3)DԪ��ԭ��ʧȥ2��4s���Ӻ�1��3d���Ӻ��ɣ�3�����ӣ���ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d64s2����Ϊ26��Ԫ������
(4)��������Ҫ������д�������Ų�ʽ��
1s22s22p63s23p63d104s1����Ԫ��Ϊ29��Ԫ��Cu��
(5)s���ֻ��1��ԭ�ӹ���������ֻ������2�����ӣ���n��2������Ԫ��F��ԭ�����������Ų�ʽΪ2s22p3���ɴ˿�֪F��NԪ�أ����ݺ�������Ų����������ԭ������֪��ԭ�ӵĺ�������е�2p�ܼ�������ߡ�


 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ָ����Ӧ�����ӷ���ʽ��ȷ����(����)
A��Cu����ϡHNO3��Cu��2H����NO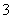 ===Cu2����NO2����H2O
===Cu2����NO2����H2O
B��(NH4)2Fe(SO4)2��Һ�����NaOH��Һ��Ӧ��Fe(OH)2��Fe2����2OH��===Fe(OH)2��
C����CH3COOH�ܽ�CaCO3��CaCO3��2H��===Ca2����H2O��CO2��
D����NaAlO2��Һ��ͨ�����CO2��Al(OH)3��CO2��AlO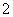 ��2H2O===Al(OH)3����HCO
��2H2O===Al(OH)3����HCO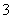
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������������йص���(����)
��NH3���ۡ��е�Ȣ�A������Ԫ���⻯��ĸߡ���С���ӵĴ���������Ժ�ˮ������Ȼ��ܡ��۱����ܶȱ�Һ̬ˮ���ܶ�С�������ص��ۡ��е�ȴ���ĸߡ������ǻ���������ۡ��е�ȶ��ǻ�������ĵ͡���ˮ���Ӹ����º��ȶ�
A���٢ڢۢܢݢ� B���٢ڢۢܢ�
C���٢ڢۢ� D���٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ԭ������Ϊ83��Ԫ��λ�ڣ��ٵ�5���ڣ��ڵ�6���ڣ��ۢ�A�壻�ܢ�A�壻�ݢ�B�壬������ȷ�������(����)
A���٢� B���ڢ� C���ڢ� D���٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��DΪԭ������С��18������Ԫ�أ�
��Aԭ�ӵĵ��Ӳ�������������������
��A��B����ͬһ���ڣ�B��D���γ����ӻ�����D2B���û������ˮ��Һ�Լ��ԣ�
��C�����Ӻ�������������������֮��Ϊ18��
��A��C��D����Ԫ�ص����Ӿ�����ͬ�ĵ��Ӳ��Ų����ƶ�A��D��Ϊ����Ԫ�أ�����գ�
(1)A________��B________��C________��D________��
(2)��ԭ���γɼ����ӵĵ����Ų�ʽ��
(3)����Ԫ�����Ӱ뾶�Ĵ�С˳��Ϊ______________________________��
(4)�����ӷ���ʽ��ʾD2Bˮ��Һ�ʼ��Ե�ԭ��___________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ϣ�ش��������⣺
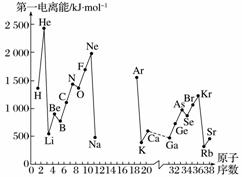
A����һ������I1��ָ��̬ԭ��X(g)���ڻ�̬ʱ��ʧȥһ�����ӳ�Ϊ��̬������X��(g)����������������ͼ�Dz���Ԫ��ԭ�ӵĵ�һ������I1��ԭ�������仯������ͼ(����12����17��Ԫ�ص��й�����ȱʧ)��
B����ͬԪ�ص�ԭ���ڷ������������ӵ�������С������ֵ��ʾ������ֵ��Ϊ�縺�ԡ�һ����Ϊ����������ɼ�ԭ�Ӽ�ĵ縺�Բ�ֵ����1.7��ԭ��֮��ͨ���γ����Ӽ�����������ɼ�ԭ�Ӽ�ĵ縺�Բ�ֵС��1.7��ͨ���γɹ��ۼ����±���ijЩԪ�صĵ縺��ֵ��
| Ԫ�ط��� | Li | Be | B | C | O | F | Na | Al | Si | P | S | Cl |
| �縺��ֵ | 1.0 | 1.5 | 2.0 | 2.5 | 3.5 | 4.0 | 0.9 | 1.5 | 1.8 | 2.1 | 2.5 | 3.0 |
(1)���������ϢAͼ��ͬ����Ԫ�ص�һ�����ܵı仯���ɣ��ƶϵ�3����Na��Ar�⼸��Ԫ���У�Al�ĵ�һ�����ܵĴ�С��ΧΪ______��Al��______(��Ԫ�ط���)��
(2)����ϢAͼ�з�����֪��ͬһ����Ԫ��ԭ�ӵĵ�һ������I1�ı仯������________________��
(3)��ϢAͼ�е�һ��������С��Ԫ�������ڱ��е�λ���ǵ�________���ڵ�________�塣
(4)���ݶԽ��߹���Be��AlԪ������������Ӧˮ������������ƣ����Ƕ�����________�ԣ�����Be(OH)2��ʾ�������ʵ����ӷ���ʽ��_________________________��
(5)ͨ�������縺��ֵ�ı仯���ɣ�ȷ��MgԪ�صĵ縺��ֵ����С��Χ________________��
(6)�����Ԫ�صĵ縺�Ժͽ����ԡ��ǽ����ԵĹ�ϵ��________________��
(7)�ӵ縺�ԽǶȣ��ж�AlCl3�����ӻ����ﻹ�ǹ��ۻ����˵�����ɲ�д���жϵķ���________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪X�Ļ�̬ԭ��L���������K���������2����Y�Ļ�̬ԭ�����������Ų�ʽΪnsnnpn��2����X�ĵ縺�Ա�Y��________(���С��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1 200 ��ʱ����Ȼ���������лᷢ�����з�Ӧ��
H2S(g)��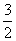 O2(g)===SO2(g)��H2O(g)����H1
O2(g)===SO2(g)��H2O(g)����H1
2H2S(g)��SO2(g)===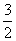 S2(g)��2H2O(g)����H2
S2(g)��2H2O(g)����H2
H2S(g)��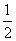 O2(g)===S(g)��H2O(g)����H3
O2(g)===S(g)��H2O(g)����H3
2S(g)===S2(g)����H4
��H4����ȷ����ʽΪ(����)
A����H4��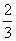 (��H1����H2��3��H3)
(��H1����H2��3��H3)
B����H4��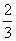 (3��H3����H1����H2)
(3��H3����H1����H2)
C����H4��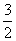 (��H1����H2��3��H3)
(��H1����H2��3��H3)
D����H4��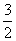 (��H1����H2��3��H3)
(��H1����H2��3��H3)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com