
2NO2+2NaOH====NaNO2+NaNO3+H2O
NO2+NO+2NaOH====2NaNO2+H2O
��1�����ڱ�״������NO��NO2�Ļ����ǡ����50 mL 2.0 mol��L-1��NaOH��Һ��Ӧ��ȫ��������NaNO2��NaNO3�����ʵ����ı�ֵΪ4��1�����ڻ��������NO������������Ϊ���٣�
��2����NO��NO2�Ļ��������NOx��ʾ���ü�Һ���գ����������Ƽ����������ٽᾧ���롣������ÿ�������Ƶijɱ�Ϊ0.16��Ԫ������ÿ���������Ƶijɱ�Ϊ0.27��Ԫ��Ŀǰ�г����ۼۣ�������ÿ��0.18��Ԫ����������ÿ��0.28��Ԫ����ÿ����22.4��109 L����״������NOx��x��1.5��0.1%�������������β������������y�����ۼۼ�ȥ�ɱ��ۣ���λ����Ԫ����x�Ĺ�ϵΪ______________������������̣�
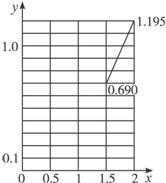
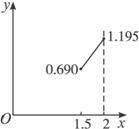
��3�����ݣ�2���ļ�����������������ϵ��������ʾ��ͼ��
��1��NO��NO2���ʵ���֮����֮��Ӧ��NaOH���ʵ�����ȣ�Ϊ0.1 mol����NO2��NO�����ʵ����ֱ�Ϊa��b��
2NO2+2NaOH====NaNO2 + NaNO3+H2O
2 2 1 1
(a-b) (a-b) 0.5(a-b) 0.5(a-b)
NO2+NO+2NaOH====2NaNO2+H2O
1 1 2 2
b b 2b 2b
![]()
a=0.07 mol b=0.03 mol
����������NO�����������0.03 mol/��0.03 mol+0.07 mol��=3/10
��2������2NOx+2NaOH====(2x-3)NaNO3+(5-2x)NaNO2+H2O
n(NOx)=106 mol����:n(NaNO2)=0.5��(5-2x)��106 mol
n(NaNO3)=0.5��(2x-3)��106 mol
y=0.02 ��Ԫ/t��0.5��(2x-3)��106 mol��85 g��mol-1��10-6 t/g+0.01 ��Ԫ/t��0.5��(5-2x)��106mol��69 g��mol-1��10-6 t/g=��1.01x-0.825����Ԫ
��3��?

��������1����NaNO3��Ϊ1������2NO2![]() NaNO2��NaNO2������3NaNO2����1.5 NO2��1.5 NO���ɵģ�NO���������
NaNO2��NaNO2������3NaNO2����1.5 NO2��1.5 NO���ɵģ�NO���������![]()
��2���ܷ�Ӧ����ʽ��?
2NOx+2NaOH====(2x-3)NaNO3+(5-2x)NaNO2+H2O
2 2x-3 5-2x
106 mol n(NaNO3) n(NaNO2)
n(NaNO2)=![]() ��106 mol
��106 mol
n(NaNO3)=![]() ��106 mol
��106 mol
y=![]() ��106 mol��69 g��moL-1��10-6 t��g-1��0.01 ��Ԫ/t+
��106 mol��69 g��moL-1��10-6 t��g-1��0.01 ��Ԫ/t+![]() ��106 mol��85 g��mol-1��10-6 t��g-1��0.02��Ԫ/t=��1.01x-0.825����Ԫ
��106 mol��85 g��mol-1��10-6 t��g-1��0.02��Ԫ/t=��1.01x-0.825����Ԫ
��3����x=1.5ʱ��y=0.690
��x=2ʱ��y=1.195



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� |
| 140�� |
| ���� |
| 140�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| 140�� |
| ���� |
| 140�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.�������᳧β���ŷţ�SO2+2NH3+H2O![]() (NH4)2SO3
(NH4)2SO3
B.�������Ṥҵβ���еĵ��������NO2+NO+2NaOH![]() 2NaNO2+H2O
2NaNO2+H2O
C.��ȡCuSO4��Cu+2H2SO4(Ũ)![]() CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O
D.��CuSO4��2Cu+O2![]() 2CuO��CuO+H2SO4(ϡ)
2CuO��CuO+H2SO4(ϡ)![]() CuSO4+H2O
CuSO4+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ɫ��ѧ��Ҫ��Ӽ����ϡ���������ƿ��еĻ�ѧ��Ӧ��ʹ�価���ܼ��ٶԻ����ĸ����á����л�ѧ��Ӧ�У���������ɫ��ѧ�������
A���ð�ˮ�������᳧��β����SO2+2NH3+H2O=(NH4)2SO3
B����ȥ���Ṥҵβ���еĵ��������NO2+NO+2NaOH=2NaNO2+H2O
C.������ͭ��Cu+2H2SO4=CuSO4+SO2��+2H2O
D��������ͭ��2Cu+O2=2CuO CuO+H2SO4(ϡ)=CuSO4+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)������������Ϊ������ȼ�ϣ��ڲ����������Ĺ����в�����CO��NO���ж����壬���������㣬��Ҫ��������Ⱦ����________�����������㣬��Ҫ�������ж�������______________��
(2)����CO��NO�����������Ӱ�죺________________________________________________��
(3)̸һ̸����β����Ⱦ��Ի�����ɵ�Ӱ��________________________________________��
(4)���ܷ�Ӧ�õ�Ԫ�صĵ��ʺͻ�����֮���ת����ϵ����Ƴ�ȥ����β���е���������(NO��NO2)�����۷���?_________________________________________________________________
(5)���Ƴ��п�����Ⱦ�ķ���������( )
A.��������Դ B.ʹ�õ綯�� C.ֲ������ D.���Ϻ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com