
����Ŀ��25��ʱ�� c mol��L��1CH3COOH ��Һ��ˮϡ�ͣ� ��Һ�� CH3COOH �� CH3COO�������и�����ռ�����ʵ�������(��)����Һ pH �仯�Ĺ�ϵ��ͼ��ʾ������˵������ȷ����
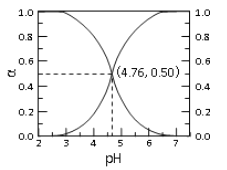
A. ��ͼ��֪�� 25��ʱ����� Ka=10-4.76
B. c mol��L��1CH3COOH ��Һ��ˮϡ���У� ��(CH3COOH)���� c(CH3COO��)Ҳһ������
C. �� pH��4.76 ����Һ��ͨ�� HCl�� ��(CH3COOH)������(CH3COO��)��С�� ��(CH3COOH)+��(CH3COO��)=1
D. ����ͼ��������������һ������Ӧ����Һ�У����� c(CH3COO��)+c(OH��)=c(H+)
���𰸡�B
��������A. ��ͼ��֪��pH=4.76ʱ����(CH3COOH)=c(CH3COO��) ������25��ʱ����� Ka=10-4.76��A��ȷ��B. c mol��L��1CH3COOH ��Һ��ˮϡ���У�CH3COOH�ĵ���ƽ�������ƶ���������(CH3COOH)��С�� c(CH3COO��)������B����ȷ��C. �� pH��4.76 ����Һ��ͨ�� HCl��HCl����ʹ��Һ��c(H+)����CH3COOH�ĵ���ƽ�������ƶ� ��������(CH3COOH)������(CH3COO��)��С�����������غ��֪�� ��(CH3COOH)+��(CH3COO��)=1��C��ȷ��D. ���������غ��֪������ͼ��������������һ������Ӧ����Һ�У����� c(CH3COO��)+c(OH��)=c(H+)��D��ȷ������ѡB.


 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д� ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���п�ѧ�������о���̼��һ��������Ӧ������������
C��s��+2NO��g��![]() CO2��g��+N2��g����H
CO2��g��+N2��g����H
(1)ʵ�鷽���������д�ʩ�������ü�����߷�Ӧ��������ʹ�ô�����߷�Ӧ��������ʹ�ü�ѹ���NOת��������ʹCO2ת���ɸɱ�����ϵ�����룬���NO��ת����������Ϊ���е���_________��
(2)�����Ӧ��ƽ�ⳣ������ʽ��_________________��
(3)�ں��ݺ����ܱ������У���ѧ�ҵõ�����ʵ������
ʱ�䣨min�� | Ũ�ȣ�mol/L�� | ||
NO | N2 | CO2 | |
0 | 0.100 | 0 | 0 |
10 | 0.058 | 0.021 | 0.021 |
20 | 0.040 | 0.030 | 0.030 |
30 | 0.040 | 0.030 | 0.030 |
��Ӧ�ڸ��¶��µ�ƽ�ⳣ��K=_________________��
(4)����(3)��ʵ����30minʱ��ʼ���£�36minʱ��ƽ�⣬���NO��ת���ʱ�Ϊ50%����÷�Ӧ����H_______0����������������������=�������жϵ�������___________________��
(5)����ѧ����30min��ı���ijһ��������Ӧ���е�40minʱ��ƽ��Ũ�ȷֱ�Ϊc��NO��=0.032mol/L��c��N2��=0.034mol/L��c��CO2��=0.017mol/L����ı������������______���жϵ�������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������������ȵ������ҵ��µĴ�л�����ۺ������Ը�Ѫ��Ϊ��Ҫ��־���������������ʳƷ��ȱ���˶������ᵼ������
��1��Ѫ����ָѪҺ�е������ǣ������й�˵����ȷ����______________��
A�������Ƿ��ӿɱ�ʾΪC6��H2O��6����ÿ�������Ƿ��Ӻ���6��H2O����
B������������ǻ�Ϊͬ���칹��
C�����������ǽϸߣ��������Ƶ�������ͭ���������Һ�е�������
D������ˮ������ղ����ǰ�����
��2�������˲��ɹ������ƣ�����һ���̶�������ȵ�ϸ�����ˡ����ƾ��ڸ����ڿ�ת�����л���A��A��ʵ�������£�
��ͨ��ʵ����A����Է�������Ϊ60��
��A��C��H��O����Ԫ����ɣ�������ֻ�����������͵���ԭ�ӣ������������͵���ԭ�Ӹ�����Ϊ1��3
��A����ƾ���һ�������������з�����ζ�����ʡ�
������A�ֳ�Ϊ______________���ṹ��ʽΪ____________________��
A����Ҫ��ѧ�����У��û�ѧ����ʽ��ʾ����a____________________________________��
b___________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڹ��ۼ���˵����ȷ���ǣ� ��
A.��Ȳ�����к���5��������2������
B.�Ҵ�������O-H���ļ���ǿ��C-H���ļ���
C.���������ֻ���м��Լ������зǼ��Լ�
D.���Ӿ����й��ۼ�����Խ�÷��Ӿ�����۵�ͷе�һ��ҲԽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NaBH4��H2O2��ԭ�ϵ�ȼ�ϵ�أ��������վ�ͨ�����ǡ���ظ������ϲ���Pt/C���������ϲ���MnO2���乤��ԭ������ͼ��ʾ������˵����������

A. ��طŵ�ʱNa+��a��������b����
B. �缫b����Pt/C���ü���Һ��pH����
C. �õ��a���ķ�ӦΪBH4-+8OH--8e-===BO2-+6H2O
D. ����ܷ�Ӧ��BH4- + 4H2O2 === BO2- + 6H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����ʵ��С����Ƶ�����ʵ�����������ǣ� ��
A. 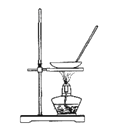 ����NH4Cl������Һ�Ʊ�NH4Cl����
����NH4Cl������Һ�Ʊ�NH4Cl����
B. 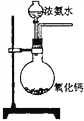 ʵ�����Ʊ���������
ʵ�����Ʊ���������
C. 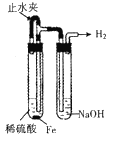 �Ʊ����۲�����������
�Ʊ����۲�����������
D. 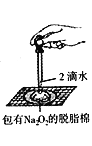 ֤������������ˮ��Ӧ����
֤������������ˮ��Ӧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ζ����ǻ�ѧ�����������ⶨ����Ũ�ȵ�һ����Ҫ��ʵ�鷽������ʳƷ��ҵ��ҩƷ���졢��ױƷ��ҵ�ȵȶ����㷺Ӧ�á��л��������ұ�(GB2760��2011)�涨���Ѿ���SO2���ʹ����Ϊ0.25 g��L��1��ij��ȤС������ͼװ��(�г�װ����)�ռ�ij���Ѿ���SO2�������京�����вⶨ��
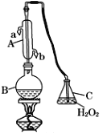
��1������A��������________��ˮͨ��A�Ľ���Ϊ________��B�м���300.00 mL���Ѿƺ��������ᣬ����BʹSO2ȫ���ݳ�����C��H2O2��ȫ��Ӧ��
��2����ȥC�й�����H2O2��Ȼ����0.090 0 mol��L��1NaOH����Һ���еζ������ζ��յ�ʱ��Һ��pH��8.8����ѡ���ָʾ��Ϊ________���ζ��յ�����Ϊ_______________��
��3���ζ����յ�ʱ������NaOH��Һ25.00 mL�������Ѿ���SO2����Ϊ��________g��L��1��
��4���òⶨ���������ʵ��ֵƫ�ߣ�����ܵ�ԭ����_________________���ڲ��ı�װ�õ�����£���θĽ���______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������з�Ӧ�У�HNO3�ȱ��ֳ������ԣ��ֱ��ֳ����Ե���
A. 3Cu��8HNO3(ϡ) =3Cu(NO3)2��2NO����4H2O��
B. CuO��2HNO3=Cu(NO3)2��H2O
C. 4HNO3(Ũ) ![]() 4NO2����O2����2H2O
4NO2����O2����2H2O
D. H2S��2HNO3(Ũ) ![]() S����2NO2��2H2O
S����2NO2��2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪a��b��c��d����Ԫ�ؾ�Ϊ����������Ԫ�أ�ԭ���������ε�����cԪ�صĵ��ʻ�cԪ�صĻ������ɫ��Ӧ�Ի�ɫ��a��b��ԭ�ӿ��γɳ����³�Һ̬�ĵ�X��Y���ֻ��������X��a��bԭ�Ӹ���֮��Ϊ2:1,Y��a��bԭ�Ӹ���֮��Ϊ1:1������a��b��c��d��ʾ���ǵĵ��ʣ�����֮�������·�Ӧ��

������֮�䷴Ӧ����+Z��X + d����+Y ��M��
M����������״Һ�壬����Ũ��Һ�ڳ����¿����������������䱣�档����Ũ��Һ����һ�����Ũ��Һ��������ըҩ��MҲ���������ᣬM����������Ǧ���ء�
(1)д��C��Ԫ�ط���: _________ ��Y�ĵ���ʽ:_______________��
(2)��30%��Y��ˮ��Һ�м���MnO2,�д���b�ĵ�������ų�,����Y��MnO2�Ļ��Һ�м���ϡH2SO4��MnO2����ԭ��Mn2+ ,��Ȼ��������b�ĵ�������,д�������ӷ���ʽ___________________________��
(3)��ʢ��NaOH��Y�Ļ��Һ���ձ��л�������Cl2���������ͱ�ը��������NaOH��������_________����Ӧ���ӷ���ʽ ____________________________��
(4)��b��c��d����Ԫ�ؿ��γ���ѧ��ѧ�����������α��������죬����Է�����������������+M����+����+d��+X�������ˮ��Һ�е��������Ҳ��d���ʵĵ���ɫ������д�����M��ˮ��Һ�л�ѧ����ʽ___��
(5)�ɺ�M������ͬԪ����ɵĻ�����A�ķ���ʽΪa2d2b8.��ҵ�ϵ��50%��M��ˮ��Һ���Ƶ�A�������ķ�ӦΪ:2M![]() A+a2��,д�������缫��Ӧ����ʽ��__________����A��Һ����ϡ���ֿ��Ƶ�Y��
A+a2��,д�������缫��Ӧ����ʽ��__________����A��Һ����ϡ���ֿ��Ƶ�Y��
(6)����c��d������֮��ķ�Ӧ�������һ��ԭ��ط�Ӧ��2C+d![]() C2d,���������ĵ缫��ӦʽΪ��____��
C2d,���������ĵ缫��ӦʽΪ��____��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com