
 | 1.01��105Pa | 5.05��105Pa | 1.01��106Pa |
| 420�� | 0.9961 | 0.9972 | 0.9984 |
| 520�� | 0.9675 | 0.9767 | 0.9852 |
| 620�� | 0.8520 | 0.8897 | 0.9276 |
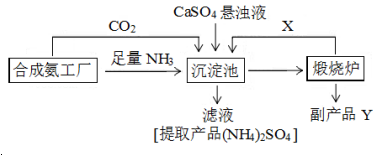
���� ��ҵ�ϳɰ��ķ�Ӧԭ��ΪN2+3H2 $\frac{\underline{\;\;����\;\;}}{���¸�ѹ}$2NH3����NH3��CO2ͨ��������в�����CaSO4�����ɣ�NH4��2SO4��CaCO3������CaSO4+CO2+2NH3+H2O=CaCO3��+��NH4��2SO4��CaCO3������¯�зֽ�����CO2��CaO��CO2��ѭ��ʹ�ã�
��1��A�������Ϊǿ����ʣ�д��=����
B��ˮ������Ϊ笠����ӣ�����ʽ��NH4++H2O?NH3•H2O+H+��
C�����ݵ���غ㣺c��NH4+��+c��H+���T2c��SO42-��+c��OH-����
D����Ũ�ȴ�С��c��NH4+����c��SO42-����c��H+����c��NH3•H2O����c��OH-����
��2������SO2��ת���ʼ������ɱ���ѡ�����¶ȶԸ÷�Ӧ��Ӱ�������
��3������v=$\frac{��c}{��t}$���㣻
��4��������ΪNH3��CO2ͨ��������в�����CaSO4�����ɣ�NH4��2SO4��CaCO3������¯������CaCO3�õ�����ƷCaO��
��5��������ͼ��������Ӧ������������ѭ�����ã���Ӧ������ȫת��Ϊ�����������ɫ��ѧ�������ܳ��������Դ��
��� �⣺��1��A�������Ϊǿ����ʣ����뷽��ʽΪ����NH4��2SO4=2NH4++SO42-��A����
B��ˮ������Ϊ笠����ӣ�ˮ�����ӷ���ʽ��NH4++H2O?NH3•H2O+H+��B��ȷ��
C�����ݵ���غ㣺c��NH4+��+c��H+���T2c��SO42-��+c��OH-����C����
D����Ũ�ȴ�С��c��NH4+����c��SO42-����c��H+����c��NH3•H2O����c��OH-����D��ȷ��
�ʴ�Ϊ��BD��
��2�����ݱ���֪����Ϊ�¶����ߣ�ת���ʼ�С��SO2��O2�ķ�Ӧ�Ƿ��ȷ�Ӧ������ѡ����¶�Ϊ420�棬��1.01��105PaʱSO2��ת�����Ѿ��ܴ�������ѹǿ��SO2��ת������߲��������������ɱ�������ѡ��1.01��105Pa����Ϊ�¶����ߣ�ת���ʼ�С��SO2��O2�ķ�Ӧ�Ƿ��ȷ�Ӧ�����¶����ߣ��÷�Ӧ��ƽ�ⳣ����С��420��ʱ��ƽ�ⳣ����520��ʱ��ƽ�ⳣ����
�ʴ�Ϊ��420�桢1.01��105Pa������
��3������SO3�����ʵ���Ϊ1.2mol����Ӧ������Ϊ0.6mol��ǰ12������������ʾ��Ӧ����v��O2��Ϊ$\frac{0.6mol��2L}{12min}$=0.025mol/��L��min����
�ʴ�Ϊ��0.025mol/��L��min����
��4����NH3��CO2ͨ��������в�����CaSO4�����ɣ�NH4��2SO4��CaCO3������CaSO4+CO2+2NH3+H2O=CaCO3��+��NH4��2SO4��CaCO3������¯�зֽ�����CO2��CaO��
�ʴ�Ϊ�������ƣ�CaSO4+CO2+2NH3+H2O��CaCO3��+��NH4��2SO4��
��5��������ͼ��������Ӧ������������ѭ�����ã�����ѭ�����õ�������CO2����Ӧ������ȫת��Ϊ�����������ɫ��ѧ�������ܳ��������Դ�������ŵ������ɵ�CO2��ѭ��ʹ�ã�CaO���Ʊ�����ƣ�û�з������ɣ�����Ⱦ�����ʲ�����
�ʴ�Ϊ��������CO2ѭ��ʹ�ã����ʳ�����ã�����Ʒ���ã�����Ⱦ�����ʲ�����
���� ���⿼�����ʵ��Ʊ�ʵ�鷽������ƣ��漰��ѧƽ����ƶ���֪ʶ�㣬��Ŀ�Ѷ��еȣ�����ע��������ʵ����ʣ����ջ�����������Լ���ҵ���������ǽ���Ĺؼ���ע�������Ϣ��ѧϰ����Ӧ�ã�


 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������Ϊ���Ӽ�Ĺ��ۼ����� | |
| B�� | ���ɵ��ʷ��ӵ���һ�����л�ѧ�� | |
| C�� | ���ӻ�������ܺ����ۼ������ۻ������п��ܺ����Ӽ� | |
| D�� | ���������У��������Ӻ������ӵľ������������⣬�����ڵ�������ӡ�ԭ�Ӻ���ԭ�Ӻ�֮����ų����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��� | ���ʣ����ʣ� | �����Լ� | ���뷽�� |
| A | ���飨��ϩ�� | ��ˮ | ϴ�� |
| B | ����������Һ������������Һ�� | ������̼ | ���� |
| C | ��ϩ��SO2�� | ����KMnO4 | ϴ�� |
| D | CO2��HCl�� | ����Na2CO3��Һ | ϴ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �밴Ҫ��ش������йص��Ļ����������ط�Ӧ�����⣺
�밴Ҫ��ش������йص��Ļ����������ط�Ӧ�����⣺| n��NO��/mol | n��CO��/mol | n��N2��/mol | n��CO2��/mol | |
| ��ʼ | 1.2 | 1.0 | 0 | 0 |
| 2minĩ | 0.4 | |||
| 4minĩ | 0.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 44gCO2��N2O�Ļ�����к��е���ԭ����Ϊ1.5NA | |
| B�� | 2L0.5mol•L-1������������Һ�к��е�HSO3-������ΪNA | |
| C�� | 0.5molCH5+�к��еĵ�����ĿΪ5NA | |
| D�� | �����£�1L0.5mol•L-1Ba��OH��2��Һ��ˮ�����OH-����Ϊ0.1NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ۢޢ� | B�� | �٢ݢ� | C�� | �ڢۢ� | D�� | �ܢݢޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
| ������ | K+��Na+��Ba2+��NH4+ |
| ������ | CH3COO-��Cl-��OH-��SO42- |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com