
��4�֣���0.1 g MnO2��ĩ����50 mL H2O2��Һ�У��ڱ�״��
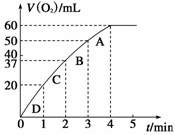 �·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ�����ͷ�Ӧ���ʱ仯��
�·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ�����ͷ�Ӧ���ʱ仯��
ԭ�� ������H2O2�ij�
ʼ���ʵ���Ũ��Ϊ ��(������λ��Ч����)
��8�֣�һ���¶��£���3 molA�����1mol B����ͨ��һ�ݻ��̶�Ϊ2L���ܱ������У��������·�Ӧ��3A(g)��B(g) ![]() xC(g)������д���пհף�
xC(g)������д���пհף�
��1����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L����1min�ڣ�B��ƽ����Ӧ����Ϊ ��XΪ ��
��2������Ӧ��2min�ﵽƽ�⣬ƽ��ʱC��Ũ�� 0.8mol/L������ڣ�С�ڻ���ڡ�����
��3������֪��ƽ��ʱ���������ڻ��������ѹǿΪp�����������ʼѹǿΪp0��������p0��p����ʾ��ƽ��ʱ��Ӧ��A��ת���ʧ�(A)Ϊ ��
��4���ܹ�˵���÷�Ӧ�ﵽƽ��ı�־�ǣ� ��
A.�����ڻ��������ܶȱ��ֲ��� B.v(A)=3v(B) C.A��B��Ũ��֮��Ϊ3:1
D.��λʱ��������3n molA��ͬʱ����n molB E.��������ƽ����Է�������
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������⡣
(1)��֪M����Է�������Ϊ227����C��H��O��N����Ԫ����ɣ�C��H��N��������������Ϊ15.86%��2.20%��18.50%����M�ķ���ʽ��________________��D��˫ԭ�ӷ��ӣ���Է�������Ϊ30����D�ķ���ʽΪ________________��
(2)��֬A������;���ɵõ�M��

ͼ�Тڵ���ʾ��
C2H5OH��HO��NO![]() C2H5O��NO2��H2O
C2H5O��NO2��H2O
���� ��������
��Ӧ�ٵĻ�ѧ����ʽ��__________________________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��__________________________________________________��
(3)C��B��������һ�������·�Ӧ���ɵĻ������Է�������Ϊ134��д��C���п��ܵĽṹ��ʽ��________________________________________________________________��
(4)����0.1 mol B�������Ľ����Ʒ�Ӧ����������_______________g�����ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������⣺
(1)��֪M����Է�������Ϊ227����C��H��O��N����Ԫ����ɣ�C��H��N��������������Ϊ15.86%��2.20%��18.50%����M�ķ���ʽ��__________��D��˫ԭ�ӷ��ӣ���Է�������Ϊ30����D�ķ���ʽΪ__________��
(2)��֬A������;���ɵõ�M��

ͼ�Тڵ���ʾ��
C2H5OH+HO��NO![]() C2H5O��NO2+H2O
C2H5O��NO2+H2O
���� ��������
��Ӧ�ٵĻ�ѧ����ʽ��_________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��_________________________________��
(3)C��B��������һ�������·�Ӧ���ɵĻ������Է�������Ϊ134��д��C���п��ܵĽṹ��ʽ__________________________________________________________________��
(4)����0.1 mol B�������Ľ����Ʒ�Ӧ����������___________g�����ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��֪M����Է�������Ϊ227����C��H��O��N����Ԫ����ɣ�C��H��N��������������Ϊ15.86%��2.20%��18.50%����M�ķ���ʽ��________��D��˫ԭ�ӷ��ӣ���Է�������Ϊ30����D�ķ���ʽΪ________��
(2)��֬A������;���ɵõ�M��

ͼ8-6
ͼ�Тڵ���ʾ��
C2H5OH+HO��NO2![]() C2H5O��NO2+H2O
C2H5O��NO2+H2O
��Ӧ�ٵĻ�ѧ����ʽ��_______________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��_______________________________________��
(3)C��B��������һ�������·�Ӧ���ɵĻ������Է�������Ϊ134��д��C���п��ܵĽṹ��ʽ_______________________________��
(4)����0.1 mol B�������Ľ����Ʒ�Ӧ����������________g�����ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��㶫ʡտ��һ�и�����ѧ��10�·��¿������ۣ���ѧ�� ���ͣ������
��0.1 mol��þ�������������100mL 2mol/L H2SO4��Һ�У�Ȼ���ٵμ�1 mol/L NaOH��Һ����������ش�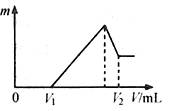
��1��д�������йص����ӷ���ʽ��
��2�����ڵμ�NaOH��Һ�����У���������m�����NaOH��Һ�����V�仯��ͼ��ʾ����V1��160mLʱ���������ĩ��n(Mg)�� mol��
V2�� mL����������������� g��
��3�����ڵμ�NaOH��Һ�����У���ʹMg2����Al3���պó�����ȫ�������NaOH��Һ�����V(NaOH)= mL��
��4�����������Ϊ0.1 mol������Mg�۵����ʵ�������Ϊa����100 mL 2 mol/L�������ܽ�˻������ټ���450 mL 1mol/L��NaOH��Һ�����ó�������Al(OH)3�������������a��ȡֵ��Χ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��㶫ʡ������ѧ��10�·��¿������ۣ���ѧ�� ���ͣ������
��0.1 mol��þ�������������100mL 2mol/L H2SO4��Һ�У�Ȼ���ٵμ�1 mol/L NaOH��Һ����������ش�

��1��д�������йص����ӷ���ʽ��
��2�����ڵμ�NaOH��Һ�����У���������m�����NaOH��Һ�����V�仯��ͼ��ʾ����V1��160mLʱ���������ĩ��n(Mg)�� mol��
V2�� mL����������������� g��
��3�����ڵμ�NaOH��Һ�����У���ʹMg2����Al3���պó�����ȫ�������NaOH��Һ�����V(NaOH)= mL��
��4�����������Ϊ0.1 mol������Mg�۵����ʵ�������Ϊa����100 mL 2 mol/L�������ܽ�˻������ټ���450 mL 1mol/L��NaOH��Һ�����ó�������Al(OH)3�������������a��ȡֵ��Χ�ǣ� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com