
��1��ij���������һ����̬������һ����̬ϩ����ɣ���ͬ��ͬѹ�£�������������������ܶ�Ϊ13���ڱ�״���£���56.0L�������ͨ��������ˮ����ˮ��������35.0g���������ṹʽΪ ______ ��������������ϩ�����ʵ���֮��Ϊ_______________��
��2��ij�л���A����Է�������Ϊ62��Ϊ��һ���ⶨA�Ļ�ѧʽ����ȡ6.2 g A��ȫȼ�գ��õ�������̼��ˮ�������������Ⱥ�ͨ��������Ũ����ͼ�ʯ�ң����߷ֱ�����5.4 g��8.8 g (����ÿ����Ӧ����ȫ)�����л����ʵ��ʽ��_____ ������ʽ��____ ____��
��ÿ��2�֣���1��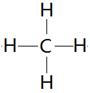 ��
3:1 ��2�� CH3O �� C2H6O2
��
3:1 ��2�� CH3O �� C2H6O2
��������
�����������1����ͬ��ͬѹ�£�������������������ܶ�Ϊ13����˵�����������Է���������26����Է�������С��26��������ֻ�м��飬���Ի������һ�����м��顣�ڱ�״���£�56.0L�����������ʵ�����56L��22.4L/mol��2.5mol���������ˮ����Ӧ������ˮ���ӵ���������ϩ�������������Լ����������2.5mol��26g/mol��35.0g��30g�����Լ�������ʵ�����30g��16g/mol��1.875mol������ϩ�������ʵ�����2.5mol��1.875mol��0.625mol���������������ϩ�����ʵ���֮��Ϊ3:1��
��2��6.2gA�����ʵ�����0.1mol����ȫȼ�����ɵ�ˮ��5.4g��CO2��8.8g������ߵ����ʵ����ֱ���0.3mol��0.2mol�����Ը���̼��ԭ���غ��֪��A�����к���̼��ԭ�ӵĸ����ֱ���2����6�������е���ԭ�Ӹ����ǣ�62��24��6����16��2�������ʽ��CH3O������ʽ��C2H6O2��
���㣺�����л��ﻯѧʽȷ�����й��жϺͼ���
�����������ǻ���������Ŀ��飬���������ǿ��ּ���������������ɺ��ܽ�����������������ڵ���ѧ����ѧϰ��Ȥ��ѧϰ�����ԣ����ѧ����Ӧ��������ѧϰЧ�ʡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������ʡĵ����һ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��1��ij���������һ����̬������һ����̬ϩ����ɣ���ͬ��ͬѹ�£�������������������ܶ�Ϊ13���ڱ�״���£���56.0L�������ͨ��������ˮ����ˮ��������35.0g���������ṹʽΪ ______ ��������������ϩ�����ʵ���֮��Ϊ_______________��
��2��ij�л���A����Է�������Ϊ62��Ϊ��һ���ⶨA�Ļ�ѧʽ����ȡ6.2 g A��ȫȼ�գ��õ�������̼��ˮ�������������Ⱥ�ͨ��������Ũ����ͼ�ʯ�ң����߷ֱ�����5.4 g��8.8 g (����ÿ����Ӧ����ȫ)�����л����ʵ��ʽ��_____ ������ʽ��____ ____��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com