
(10��) (1)��һ�������£��ݻ�Ϊ 10 L�ܱ������з�����Ӧ��
CH4(g)+H2O(g)
 CO(g)+3H2(g)����H��0
CO(g)+3H2(g)����H��0
��1.0 mol CH4��2.0 mol H2O(g)ͨ����ܱ����� 3 sʱ��0.1 mol CO���ɣ���3 s�ڸ÷�Ӧ��ƽ������v(H2)= ��
(2)��ѹǿΪ0.1
MPa������,�ݻ�ΪV Lij�ܱ�������a
mol CO�� 2a mol H2�ڴ��������·�Ӧ���ɼ״���CO(g)+2H2(g)  CH3OH(g)��CO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��
CH3OH(g)��CO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��
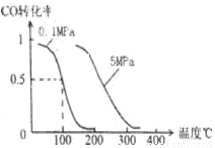
�ٸ÷�Ӧ�� ��Ӧ(����ȡ������ȡ�)��
��150��ʱ�÷�Ӧ��ƽ�ⳣ��K V2��a2(���������������)��
�����¶��ݻ����������£�����ܱ�����������a mol CO�� 2a mol H2����b mol CH3OH(g)����ﵽ��ƽ��ʱ��CO��ת���� (���������С�����䡱����ȷ����)��ƽ�ⳣ�� (���������С�����䡱)��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ����������������һ�и�һ��һ���¿���ѧ�Ծ����������� ���ͣ������
����5�֣�(1)��С�ձ��м���25 mL����ˮ��������________�������м���5��___________�������ֱ����Һ��__________ɫ�������Ƶ�Fe(OH)3���塣
��2����֪����������������������������������Fe3�����ɵġ��ֽ����������������ʹ����ֱ����10��9��10��7m����������ˮ�У���������Һ�м���___________��______________�������Ƶ�Fe(OH)3���塣�������ͬ���ʣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ɹŰ�����ѧ��һ12���¿���ѧ�Ծ����������� ���ͣ������
��10 �֣�(1)���������Ϊ ����������; ������ˮ�еĵ��뷽��ʽ�� ��
(2)��ȥNa2CO3��ĩ�л����NaHCO3������������������������ѧ����ʽΪ�� ����������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ�ʹ�һ�и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
(10��)��.��1�֢�ȥ��̬ԭ���е�һ������ʹ֮��Ϊ��̬+1��������ʱ��������ṩ������������Ԫ�صĵ�һ�����ܡ���ͼ�����ڱ��ж����ڵ�һ���֣����е�һ��������С��Ԫ����_______. ������ĸ��
��.��6�֣��¹���������ѧ�������Ƴ���20��̼ԭ����ɵ�
������״����C20������״�ṹ��������������ι��ɣ���ͼ������ش�: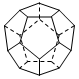
C20���ӹ���_______��������Σ�����_______����ߣ�C20��������_______ (�������).
��. ��3�֣�������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl����ṹ��ͼ��ʾ��
��1��������ÿ��Na+ͬʱ������______��Cl-��ÿ��Cl-ͬʱ������_______��Na+��
��2����������ÿ��Cl-��Χ������ӽ��Ҿ������
��Cl-����________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�ص���ѧ�߶�5��������⻯ѧ�Ծ����������� ���ͣ������
(10��)��1��AgNO3��ˮ��Һ�� ����ᡱ�����С���������ԣ�ԭ���ǣ������ӷ���ʽ��ʾ���� ������������������ ��
��2���Ȼ���ˮ��Һ�� �ԣ�����ᡱ�����С���������ԣ�ԭ���ǣ������ӷ���ʽ��
ʾ���� ;
��AlCl3��Һ���ɣ����գ����õ�����Ҫ��������� ��
(3)��֪MgCl2��6H2O�����ڿ����м���ʱ���ͷŲ��ֽᾧˮ��ͬʱ����Mg(OH)Cl����ʽ�Ȼ�þ��������MgO�������ǹ���MgCl2��6H2O���ۺ�Ӧ�ã�
��ش��������⣺
������ͼ��������Ӧ�������_______________�����м��ȡ�
��Mg(OH)2������������ܽ�ƽ�⣺Mg(OH)2(��)  Mg2����2OH��������ϵ�м��루��������ֲ�ͬ�������ʣ�_____________________________________________________ ������Mg(OH)2�ܽ⡣
Mg2����2OH��������ϵ�м��루��������ֲ�ͬ�������ʣ�_____________________________________________________ ������Mg(OH)2�ܽ⡣
����֪AnBm�����ӻ���c (Am��) n ��c (Bn��) m��ʽ��c (Am��) n��c (Bn��) m��ʾ���ӵ����ʵ���Ũ�ȣ�������ʱ�����Mg(OH)2������Һ��pHֵΪ11�������ӻ�Ϊ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ������һѧ��ģ�鿼�Ի�ѧ�Ծ� ���ͣ������
��10 �֣�(1)�ס�������Ԫ����ͬһ���ڵ�����Ԫ�أ���Ԫ�����γ��л������ҪԪ�أ���Ԫ�ص�P�Dz�����3�����ӡ�
��д����Ԫ�صĵ����Ų�ʽ________________________��
�ڼס���Ԫ�صĵ�һ�����ܹ�ϵΪ�� �ң���>��<��=������Ԫ�ؿ��γ�Ӳ�ȴ��ڽ��ʯ��һ�ֻ�����û���������______���壬�仯ѧʽΪ_____________����ʹ���ۻ������ƻ������ù�ϵΪ___________________.
��2������һֱ�������˹��̵����о����Ի�����۵ĵ��ʡ���ѧ���Ⱥ�������ϳ��˹̵�ø�Ķ���ģ�������һ���Ǻ�Mo��Fe��Sԭ�ӵ���������ṹ������ͼ��ʾ��ͼ���������߶Գƣ�����һ������Ϊ������Ľṹ��ÿ�������庬��4��Feԭ�ӡ�4��Sԭ�ӣ�����λ���������8�����㣬��ԭ�Ӽ�ֻ��һ����ѧ����

������ͼ�����������ġ�����д��(�ڡ�Ϳ��)����3��Feԭ��
������һ����������4��Feԭ�����ڵĶ������������ɵĿռ伸����Ϊ______
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com