
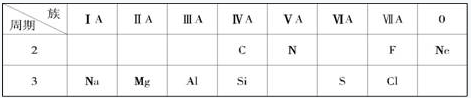
| a | | b | |||||||||||||||
| | | | | | | | | | | | | | c | d | | | |
| e | f | g | h | | | i | | ||||||||||
| | | | | | | | | j | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
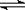 NH4Ј«Ј«OHЈЈ¬КФЕР¶ПNH3ИЬУЪЛ®єуЈ¬РОіЙµДNH3Ў¤H2OµДєПАнЅб№№КЗ____ __ (МоРтєЕ) ЎЈ
NH4Ј«Ј«OHЈЈ¬КФЕР¶ПNH3ИЬУЪЛ®єуЈ¬РОіЙµДNH3Ў¤H2OµДєПАнЅб№№КЗ____ __ (МоРтєЕ) ЎЈ
| | п® | X | Y |
| К§ИҐµЪТ»ёцµзЧУ | 519 | 502 | 580 |
| К§ИҐµЪ¶юёцµзЧУ | 7296 | 4570 | 1820 |
| К§ИҐµЪИэёцµзЧУ | 11799 | 6920 | 2750 |
| К§ИҐµЪЛДёцµзЧУ | | 9550 | 11600 |
 ЈЁ2·ЦЈ© ЈЁ2Ј©>ЈЁ2·ЦЈ© >ЈЁ2·ЦЈ© <ЈЁ2·ЦЈ©
ЈЁ2·ЦЈ© ЈЁ2Ј©>ЈЁ2·ЦЈ© >ЈЁ2·ЦЈ© <ЈЁ2·ЦЈ© ЎЈ
ЎЈ

 ФД¶БїміµПµБРґр°ё
ФД¶БїміµПµБРґр°ё
| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈєІ»Пк МвРНЈєМоїХМв
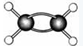 ОЄДіУР»ъОпµДЗт№чДЈРНЈЁЖдЦР
ОЄДіУР»ъОпµДЗт№чДЈРНЈЁЖдЦР ґъ±нЗвФЧУґъ±н
ґъ±нЗвФЧУґъ±н МјФЧУЈ©Ј¬ёГУР»ъОпЦРМјФЄЛШУлЗвФЄЛШµДЦКБї±Иm(C):m(H)ЈЅ ЎЈЈЁПа¶ФФЧУЦКБїC-12ЎўH-l)
МјФЧУЈ©Ј¬ёГУР»ъОпЦРМјФЄЛШУлЗвФЄЛШµДЦКБї±Иm(C):m(H)ЈЅ ЎЈЈЁПа¶ФФЧУЦКБїC-12ЎўH-l) Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈєІ»Пк МвРНЈєµҐСЎМв
| AЈ®ФЧУ°лѕ¶ AЈѕBЈѕDЈѕC | BЈ®ФЧУРтКэdЈѕcЈѕbЈѕa |
| CЈ®АлЧУ°лѕ¶ C3ЈЈѕDЈЈѕBЈ«ЈѕA2Ј« | DЈ®µҐЦКµДµзёєРФ AЈѕBЈѕDЈѕC |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈєІ»Пк МвРНЈєМоїХМв
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈєІ»Пк МвРНЈєµҐСЎМв
| AЈ®Т»ёцO4·ЦЧУУЙБЅёцO2·ЦЧУ№№іЙ | BЈ®O4КЗТ»ЦЦµҐЦК |
| CЈ®µИЦКБїµДO4єНO2є¬УРПаН¬КэДїµДФЧУ | DЈ®O4КЗТ»ЦЦ»ЇєПОп |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈєІ»Пк МвРНЈєМоїХМв
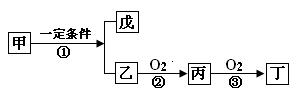
 ТТОпЦКОЄИјБПµДОўЙъОпИјБПµзіШЅб№№КѕТвНјИзНјЛщКѕЈ¬ёГФµзіШµДёєј«·ґУ¦·ЅіМКЅОЄ
ТТОпЦКОЄИјБПµДОўЙъОпИјБПµзіШЅб№№КѕТвНјИзНјЛщКѕЈ¬ёГФµзіШµДёєј«·ґУ¦·ЅіМКЅОЄ 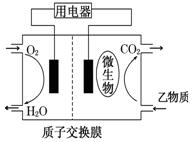
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈєІ»Пк МвРНЈєМоїХМв
 ЧУІгЈ¬µЪТ»ІгУлЧоНвІгµзЧУКэПаµИЈ»јЧФЧУµДєЛНвµзЧУКэ±ИТТФЧУєЛНвµзЧУКэЙЩ1Ј»±ыФЧУµДЧоНвІгµзЧУ
ЧУІгЈ¬µЪТ»ІгУлЧоНвІгµзЧУКэПаµИЈ»јЧФЧУµДєЛНвµзЧУКэ±ИТТФЧУєЛНвµзЧУКэЙЩ1Ј»±ыФЧУµДЧоНвІгµзЧУ КэКЗґОНвІгµзЧУКэµД2±¶Ј»¶ЎФЧУєЛµзєЙКэ±И±ыФЧУєЛµзєЙКэ¶а2ЎЈЗл»ШґрЈє
КэКЗґОНвІгµзЧУКэµД2±¶Ј»¶ЎФЧУєЛµзєЙКэ±И±ыФЧУєЛµзєЙКэ¶а2ЎЈЗл»ШґрЈє ЦКУР Ўў
ЦКУР ЎўІйїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈєІ»Пк МвРНЈєМоїХМв

Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈєІ»Пк МвРНЈєМоїХМв
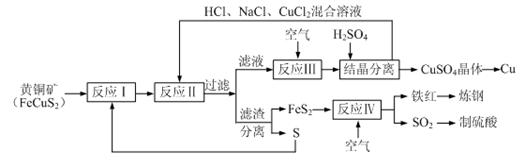
 3++
3++  Fe3Ј«+ H2OЈЁОґЕдЖЅЈ©
Fe3Ј«+ H2OЈЁОґЕдЖЅЈ©Ійїґґр°ёєНЅвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com