
̼����Ԫ�ؼ��仯�������������������������ء��Իش������й����⣺
��1��NH3��������ˮ����ˮ��Һ�׳ư�ˮ����ˮϡ��0.1mol?L-1�İ�ˮ����Һ������ˮ�������Ӷ���С���� ������ţ���
A��![]() B��
B��![]()
C��c(H+)?c(OH-) D��![]()
��2����״���£���1.12LCO2ͨ��100mL mol?L-1��NaOH��Һ�У�������Һ������Ũ���ɴ�С��˳��Ϊ ��
����Һ������Ũ�ȷ���������е�ʽ��
��c(OH-)=2c(H2CO3)+ ��
��c(H+)+c(Na+)= ��
��3������ȼ�ϵ���з����Ļ�ѧ��ӦΪ��CH4+2O2=CO2+2H2O���õ�صĵ������ҺΪH2SO4��Һ����ԭ��ع���ʱ�������Һ���������ƶ��������� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

c(N
| ||
| c(NH3?H2O) |
| c(NH3?H2O) |
| c(OH-) |
| c(OH-) |
| c(H+) |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�̼����Ԫ�ؼ��仯�������������������������ء��Իش������й����⣺
��1�� NH3��������ˮ����ˮ��Һ�׳ư�ˮ����ˮϡ��0.1mol��L��1�İ�ˮ����Һ������ˮ�������Ӷ���С����_____________������ţ�
��2����״���£���1.12LCO2ͨ��100mL1mol��L��1��NaOH��Һ�У�������Һ������Ũ���ɴ�С��˳��Ϊ_________________________________________________��
��c��OH����=2c��H2CO3��+______________________________________________��
��c��H+��+c��Na+��=___________________________________________________��
��3������ȼ�ϵ���з����Ļ�ѧ��ӦΪ��CH4+2O2=CO2+2H2O���õ�صĵ������ҺΪH2SO4��Һ����ԭ��ع���ʱ�������Һ���������ƶ���������_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡΫ�����ٹ��ִ���ѧ����12���¿���ѧ�Ծ� ���ͣ������
��10�֣�̼����Ԫ�ؼ��仯�������������������������ء��Իش������й����⣺
��1�� NH3��������ˮ����ˮ��Һ�׳ư�ˮ����ˮϡ��0.1mol��L��1�İ�ˮ����Һ������ˮ�������Ӷ���С����_____________������ţ�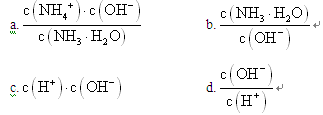
��2����״���£���1.12LCO2ͨ��100mL1mol��L��1��NaOH��Һ�У�������Һ������Ũ���ɴ�С��˳��Ϊ_________________________________________________��
��c��OH����=2c��H2CO3��+______________________________________________��
��c��H+��+c��Na+��=___________________________________________________��
��3������ȼ�ϵ���з����Ļ�ѧ��ӦΪ��CH4+2O2=CO2+2H2O���õ�صĵ������ҺΪH2SO4��Һ���� ԭ��ع���ʱ�������Һ���������ƶ���������_____________��
ԭ��ع���ʱ�������Һ���������ƶ���������_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡΫ���и���12���¿���ѧ�Ծ� ���ͣ������
��10�֣�̼����Ԫ�ؼ��仯�������������������������ء��Իش������й����⣺
��1�� NH3��������ˮ����ˮ��Һ�׳ư�ˮ����ˮϡ��0.1mol��L��1�İ�ˮ����Һ������ˮ�������Ӷ���С����_____________������ţ�
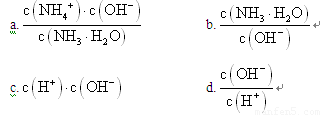
��2����״���£���1.12LCO2ͨ��100mL1mol��L��1��NaOH��Һ�У�������Һ������Ũ���ɴ�С��˳��Ϊ_________________________________________________��
��c��OH����=2c��H2CO3��+______________________________________________��
��c��H+��+c��Na+��=___________________________________________________��
��3������ȼ�ϵ���з����Ļ�ѧ��ӦΪ��CH4+2O2=CO2+2H2O���õ�صĵ������ҺΪH2SO4��Һ����ԭ��ع���ʱ�������Һ���������ƶ���������_____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com