
�ҹ�����ר�Һ�°����ڴ��£��Ľ��������ˡ������Ƽ����Ϊ�����Ƽҵ������ͻ�����ס�������������⣺
��1���������Ƽ���Ƶõġ���� (�ѧʽ)��
��2������������Ƽ��������Ҫ�Ĺ�ҵ�Ƽ�����б����У�����ȷ���� ��
| | | ��� | �����Ƽ |
| A | ԭ�� | ʳ�Ρ���������ʯ�� | ʳ�Ρ�������������̼ |
| B | ���ܵĸ����� | �Ȼ��� | �Ȼ�� |
| C | ѭ������ | ������������̼ | �Ȼ��� |
| D | ���� | ԭ���ã��豸���ӣ��ܺĸ� | ԭ�������ʸߣ��������� |

��1��Na2CO3
��2��A.C
��3��f e d c ��2�֣� B
��4�������� ����NaHCO3��Һ
��5��CO2+NH3+NaCl+H2O��NaHCO3��+NH4Cl
��6��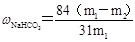 �� m1Ϊ��Ʒ������m2Ϊ���Ⱥ��Ʒ��������
�� m1Ϊ��Ʒ������m2Ϊ���Ⱥ��Ʒ��������
���������������1���������Ƽ���Ƶõġ����̼���ƣ���ѧʽΪNa2CO3��
��2��A�����ԭ���У�ʳ�Σ��Ȼ��ƣ���ʯ��ʯ��������������ʯ�ҺͶ�����̼���������������Ƽԭ���У�ʳ�Ρ�������������̼����A����B��������ܵĸ�����Ϊ�Ȼ��ƣ������Ƽ���ܵĸ������Ȼ�泥���B��ȷ��C�����ѭ�����ʣ�������������̼�������Ƽѭ�����ʣ��Ȼ��ƣ�������̼����C����D�����ԭ�ϣ�ʳ�κ�ʯ��ʯ�����ˣ���Ʒ����Ĵ��ȸߣ�����Ʒ���Ͷ�����̼�����Ի���ѭ��ʹ�ã����첽����ʺ��ڴ��ģ���������豸���ӣ��ܺĸߣ���������ȱ�㻹����ԭ��ʳ�ε�������ֻ��72%��74%�������Ƽ�����ŵ���ʹʳ�ε���������ߵ�96%���ϣ��������٣���D��ȷ����ѡAC��
��3��Aװ�����Ʊ�CO2��Bװ�����Ʊ�NH3�����ڰ�����������ˮ��Ҫ�ø���ܷ�ֹ����������CO2�е��Ȼ������廹��Ҫ��ȥ�������ȷ������˳��Ϊ(a)��(f)��(e)��(d)��(b)��(c)������CO2��ˮ�е��ܽ��С�����Ҫ���Ȳ���������Ȼ����ͨ��CO2���壬����Ӧ������Bװ���ȷ�����Ӧ��
��4��������������ˮ�����C�������θ���ܶ�����ֱ���ܵ������Ƿ���������ȥCO2�е��Ȼ�������Ӧ���ñ���NaHCO3��Һ����D��Ӧѡ�õ�Һ��Ϊ����NaHCO3��Һ��
��5��Cװ�����Ʊ�̼�����Ƶģ�����C�й��ƿ�ڲ���������ܻ�ѧ����ʽΪCO2+NH3+NaCl+H2O��NaHCO3��+NH4Cl��
��6����m1Ϊ��Ʒ������m2Ϊ���Ⱥ��Ʒ�������������̼�����Ʒֽ�ķ���ʽ��֪
2NaHCO3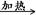 Na2CO3��H2O��CO2�� �����������١�m
Na2CO3��H2O��CO2�� �����������١�m
168g 106g 62g
X m1��m2
���X��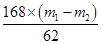
���Դ�����̼�����Ƶ����������ɱ�ʾΪ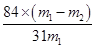 ��
��
���㣺��������Ƽ���й��жϡ������


 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��1���֣�
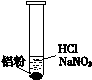
һλͬѧ�ڸ�ϰʱ��������һ��ϰ�⣺ij��ɫ��Һ�п��ܺ��С�H+��OH-��Na+��NO3-�����������ۺ�ֻ����H2���ʸ���ɫ��Һ���ܴ��������ļ������ӡ�
��1���������۲���H2��˵��������______��������ԡ���ԭ�ԡ�����
��2����ͬѧ��������H+�������ڣ���NO3-�Ͳ��ܴ������ڡ�
���ʵ��֤ʵ���£�
| װ �� | �� �� |
 | ��. ʵ���ʼ��δ���������� ��. ��һ������������ݣ�Һ���Ϸ���dz��ɫ ��. �Թܱ��ȣ���Һ���� |
| ʵ �� | �� �� | �� �� |
| ʵ��1 | ��ʪ��KI��������ֽ���ڿ����� | δ���� |
| ʵ��2 | ��ʪ��KI��������ֽ����dz��ɫ���� | ��ֽ���� |
| װ �� | �� �� |
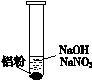 | ��. ʵ���ʼ��δ���������� ��. ��һ������������ݣ��д̼�����ζ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��12�֣���ҵ����������(��Ҫ�ɷ�ΪAl2O3��Fe2O3��)��ȡAl2O3��ұ������ԭ�ϣ������ε�ⷨ��õĴ����к�һ�����Ľ����ƺ���������Щ���ʿɲ��ô�����������ȥ��������β��������������ڸֲĶ�����������������ͼ��ʾ��
(��֪��NaCl�۵�Ϊ801�棻AlCl3��181������)
��1������Һ��ͨ�����CO2��������Ӧ�����ӷ���ʽΪ ��
��2������ǰ������������������������ʯӢɰ����ֹ����ʱ���Ƿֱ����������û���Ӧ�����µ����ʣ���������������Ӧ�Ļ�ѧ����ʽΪ ��
��3����Cl2����ͨ�������еĴ������壬�����������ϸ�����ȥ�����ݵ���Ҫ�ɷֳ�Cl2�����______����̬����ճ���������ϣ�����������γɸ����������п϶�����________��
��4�����������У�������Ϊ�����������ε��Һ����Ԫ����Ҫ��AlCl4����ʽ���ڣ��������ĵ缫��ӦʽΪ_____________��
��5���ֲĶ�������ʴ���ܻ�����ǿ����ԭ����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��15�֣�ʵ�����Է�ͭмΪԭ����ȡ��ʽ̼��ͭ��Cu2(OH)2CO3���IJ������£�
����һ����ͭм������ͭ
��ͼ��
�ý�ͷ�ι���ȡŨHNO3�����ӵ���ƿ�ڵķ�ͭм��(��ͭм����)����ַ�Ӧ����ˣ��õ�����ͭ��Һ��
���������ʽ̼��ͭ���Ʊ�
����Թ��м���̼������Һ������ͭ��Һ��ˮԡ������70�����ң���0.4 mol/L��NaOH��Һ����pH��8.5�������ã����ˣ�����ˮϴ�ӣ���ɣ��õ���ʽ̼��ͭ��Ʒ��
����������ʽ̼��ͭ����ɲⶨ
��ʽ̼��ͭ�ɱ�ʾΪ��xCuCO3 ��yCu (OH)2 ��zH2O���ɲ���������ԭ����ȷ�����䷴Ӧԭ��Ϊ��
xCuCO3 ��yCu (OH)2 ��zH2O + H2�� Cu + CO2 + H2O��δ��ƽ��
���������գ�
��1������һ�У���Ӧ��ʼʱ��ƿ�ڵ������� ��
�ø�װ����ȡ����ͭ���ô��� ��
��2��������У�ˮԡ�������������� �� (���ȡ��г�������ʯ��������)��ϴ�ӵ�Ŀ���� ��
��3�� �������У�������x��y��zĸΪϵ������ƽ������ԭ���Ļ�ѧ����ʽ��
xCuCO3 ��yCu (OH)2 ��zH2O+ H2�� Cu+ CO2+ H2O
�ڳ�ȡ24.0gij��ʽ̼��ͭ��Ʒ����ַ�Ӧ��õ�12.8 g���������4.4g������̼��7.2gˮ������Ʒ�нᾧˮ����Ϊ g����ѧʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
һλѧ����������������Ӧ��IJ������̽����
(1)�������
����1������ΪFeO��
����2�� ��
(2)��������
��ѧ��ͨ���������ϵ�֪�������������������У��������������ȶ�������������ȶ��������¼��ױ�����������������(��ɫ�ɺ�ɫ��ɺ�ɫ)��
ͨ���������Ͽ��Եó��ij�������Ϊ ��
(3)����ʵ��
��ѧ�����������װ�ý���������������Ӧ��ʵ�顣�����������ʵ�鲽�貹��������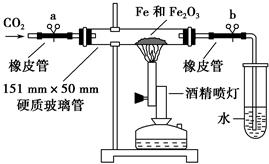
�ٰ���ͼװ�����Ӻ�����(�ݲ�װ��ҩƷ)�� ��
�ڳ�ȡ1 g��ԭ�����ۺ�5 g��������ĩ����Ͼ��Ⱥ�ƽ̯�ڲ������в���
���ɿ��������ɼУ� �����ɼ��ϵ��ɼ�a������ʼ����ҩƷ��
�ܴ�Լ4�������ң���ɫ��ĩȫ����ڣ��ټ��ϵ��ɼ�b��Ȼ��ֹͣ���ȣ��ȵ���������ȴ�����£�������ɫ��ĩ��
(4)���ṩ����ҩƷ����֤ʵ��õ��ĺ�ɫ��ĩ�ijɷ֡�������ϡ���ᡢKSCN��Һ������KMnO4��Һ���Թܡ���ͷ�ιܡ�
| ʵ�鲽�� | Ԥ������ͽ��� |
| | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�������д�����CuS����������������������ʡ�ʵ�������Ըÿ���Ϊԭ���Ʊ�CuCl2��2H2O���壬�������£�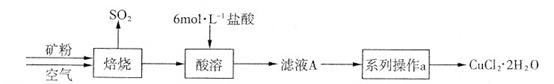
��1����ʵ�����У�����37��(�ܶ�Ϊ1��19 g��mL-1)����������500 mL 6 mol��L-1�����ᣬ��Ҫ����������Ͳ���ձ����������⣬���� �� ��
��2��������ʵ���������ϵ�в���a��������ʵ������У�����Ҫ���� (�����и��������)��
��CuCl2��Һ�д�������ƽ�⣺Cu(H2O)42+(��ɫ)+4Cl- CuCl42-(��ɫ)+4H2O��
CuCl42-(��ɫ)+4H2O��
����ʵ��֤����ҺA(��ɫ)�д�������ƽ�⣬����ҺA�⣬�����Լ��У�����Ҫ���� (�����и��������)��
a��FeCl3���� b��CuCl2���� c������ˮ
��3��ij��ѧС������ʵ�������о�CuS���յķ�Ӧ���̣��������ϵ�֪�ڿ��������±���CuSʱ�����������仯����SO2������������ͼ��ʾ��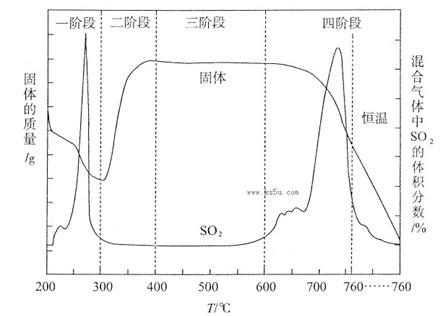
��CuS�����ڱ��չ����У���Cu2S��CuO��CuSO4��CuSO4��CuO���ɣ�ת��˳��Ϊ��
�ڢٲ�ת����Ҫ��200��300oC��Χ�ڽ��У��ò�ת���Ļ�ѧ����ʽΪ ��
��300��400oC��Χ�ڣ����������������ӵ�ԭ���� ����ͼ��ʾ�����У�CuSO4�������ȶ����ڵĽ��� (�����и��������)��
a��һ�� b������ c������ d���Ľ�
�۸û�ѧС���������װ��ģ��CuS�����������б��յ��ĽεĹ��̣�����֤��������ΪSO2��O2�Ļ���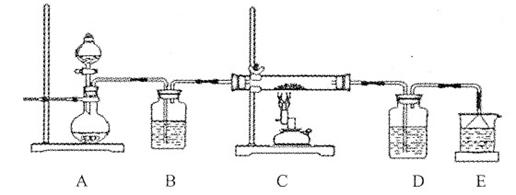
a��װ����װ��ɺ�Ӧ�������е�һ������� ��
b����Dװ���в�����ɫ����ʱ������˵�����Ľ���������ΪSO2��O2�Ļ�������Ϊװ��D��ԭ��ʢ�е���ҺΪ ��Һ��
c����ԭCuS����������Ϊl0��0 g����ʵ������У������¶���760oC���ҳ������ȣ���������ַ�Ӧ��ʯӢ�����������ù��������Ϊ8��0 g����ԭ������CuS����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ��Ŀ�ģ�̽������������ˮ��Ӧ�����Һ�μӷ�̪��Һ�ȱ�����ɫ��ԭ��
[���������]
��1�����ݹ���������ˮ��Ӧ��ԭ����2Na2O2 + 2H2O =" 4NaOH" + O2�������������ƹ�����ȫ�ܽⷴӦ�����Һ�еμӷ�̪��Ӧֻ�����������ɫ����ʵ���з��ַ�̪��������ɫ���ɴ�������µIJ��룺
A��������Ư����
B������������Ư����
C��
[ʵ�����ж�] ��������б���
| ʵ���� | 1 | 2 | 3 |
| ʵ��װ�� | 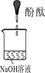 | 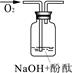 | 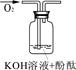 |
| ��֤���� | | C | |
| ʵ������ | ��Һ������ɫ | ||
| ʵ��˵�� | 1��2��ʵ����NaOH��Һ���� ����������ƹ��塱���������ƹ��塱�����������ƹ��塱������ˮ���Ƶġ� | ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ѧ��ȤС���������ʵ�鷽��,�ⶨij�Ѳ��ֱ��ʵ�С�մ���Ʒ��Na2CO3������������
������һ�� ��ȡһ�������Ĺ�����Ʒ,ͨ�����������غ���ȴ,����ʣ���������,���㡣
(1)����������,�����չ�����Ʒʱ�����õ�������������(������)�� 
(2)ʵ��������������ص�Ŀ����:�� ��
(3)ʵ����ȷ�����������صķ�����:�� ��
(4)�����ȹ������о���Ž�����,���õĽ����������(�ƫ����ƫС�����䡱)��
���������� ��ȡһ��������Ʒ,����С�ձ���,������ˮ�ܽ�;��С�ձ��м�������Ba(OH)2��Һ,����,ϴ��,�������,������������,���㡣
(��֪:Ba2++OH-+HC
 BaCO3��+H2O)
BaCO3��+H2O)
(1)���˲�����,�����ձ���©����,��Ҫ�õ��IJ�������Ϊ ������
(2)ʵ�����жϳ����Ƿ���ȫ�ķ������� ��
(3)ʵ����ϴ�ӳ����IJ������� ��
(4)ʵ�����жϳ����Ƿ�ϴ�Ӹɾ��ķ������� ��
���������� ����ͼ��ʾװ�ý���ʵ��: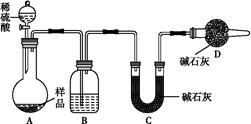
(1)Bװ������ʢ�Լ�����������;Dװ�õ�����������������������������������;��Һ©������������(��ܡ����ܡ�)���������ϡ�������ʵ�顣
(2)ʵ��ǰ��ȡ17.9 g��Ʒ,ʵ�����Cװ������8.8 g,����Ʒ��Na2CO3����������Ϊ����������
(3)���ݴ�ʵ���õ�����,�ⶨ��������,��Ϊʵ��װ�û�����һ������ȱ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������岻��ȱ�ٵ���Ԫ�أ������������壨FeSO4��7H2O����ҽҩ������Ѫ����ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����������ò�Ѫ���Ƿ���ʡ�ʵ�鲽�����£�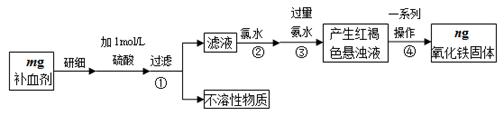
��1��ʵ�������Ѿ�ȷ��ȡ��Ũ��������500 mL 1 mol��L-1��������Һ��������ʱ�õ����������ձ���500mL����ƿ�⣬����Ҫ ��
��2������ڼ��������ˮ������Ӧ�����ӷ���ʽΪ ��
��3���������һϵ�в�������Ϊ ��ϴ�ӡ����ա���ȴ��������
��4����ʵ������ģ���ò�Ѫ������Ԫ�ص���������Ϊ ��
��5��ijͬѧ���ֲ��ֲ�Ѫ��ҩƬ���淢�ƣ�ȡ��Ʒ��ϸ������1 mol��L-1��������Һ�����ˡ�ȡ��Һ�� _______________��Һ����Һ���˵���ò�Ѫ���Ѳ��ֱ��ʡ����ɫ�����Һ�м����֭����Һ��ɫ��ȥ��˵����֭������ �ԣ����������ԭ���������ʡ�
��6�����м��飨5������Һ���Ƿ���Fe2+Ӧѡ�õ��Լ��� ��
| A��KSCN��Һ | B������KMnO4��Һ | C������ | D��FeCl3��Һ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com