
| A�� | X��Y��Z��W��M��N��ԭ�Ӱ뾶�Ĵ�С��ϵΪ��N��M��W��Y��Z��X | |
| B�� | X��Y��Z��W����Ԫ�ؿ��γɻ�ѧʽΪX7Y2ZW2�Ļ����� | |
| C�� | ��Ԫ��Y��Z��ȣ�Ԫ��W�γɵļ��⻯�����ȶ�������Ϊ����Ӽ������� | |
| D�� | ��X��W��M��N����Ԫ���γɵĻ����������ˮ��ˮ��Һһ�������� |
���� Z2X4��������ƽ�����ȼ�ϣ�X2W2Ϊ������18���ӻ����������֪Z2X4ΪN2H4��X2W2ΪH2O2������X��Y��Z��W��M��N��ԭ��������������X��Mͬ���壬W��Nͬ���壬֪X��Z��W��M��N�ֱ�ΪH��N��O��Na��S����X��Y��Z��W��ԭ������֮��Ϊ22֪YΪC��
��� �⣺Z2X4��������ƽ�����ȼ�ϣ�X2W2Ϊ������18���ӻ����������֪Z2X4ΪN2H4��X2W2ΪH2O2������X��Y��Z��W��M��N��ԭ��������������X��Mͬ���壬W��Nͬ���壬֪X��Z��W��M��N�ֱ�ΪH��N��O��Na��S����X��Y��Z��W��ԭ������֮��Ϊ22֪YΪC��
A��ͬ���ڴ�����ԭ�Ӱ뾶��С��ͬ������ϵ���ԭ�Ӱ뾶������֪������Ԫ��ԭ�Ӱ뾶����ΪNa����A����
B��X��Y��Z��W�ֱ�ΪH��C��N��O�����ݲ����Ͷȿ���֪����̼ԭ�ӣ�һ����ԭ����������7����ԭ�ӣ���CH2OH-CHOH-NH2�ȣ���B��ȷ��
C�����ۻ�������ȶ���������أ����빲�ۼ������йأ���C����
D��X��W��M��N����Ԫ��ΪH��O��Na��S���γɵĻ�����ܶ࣬��������ˮ�����Եģ���NaHSO3��NaHSO4��Ҳ�г����Եģ���â��Na2SO4•10H2O����D����
��ѡB��
���� ���⿼��ԭ�ӽṹ��Ԫ�������ɵĹ�ϵ����Ŀ�Ѷ��еȣ�����Bѡ��Ҫ����л���ѧ֪ʶ�����жϣ�Ҫ��ϸߣ�


 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | Cl2 | Br2 | I2 | HCl | HBr | HI | H2 |
| ������kJ�� | 243 | 193 | 151 | 432 | 366 | 298 | 436 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 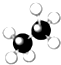 | B�� | 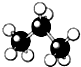 | C�� | 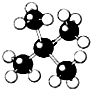 | D�� | 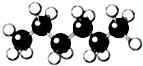 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ĭ������н�Al2��SO4��3��NaHCO3����Һ��Ϻ����������ĭ����������� | |
| B�� | KNO3��KClO3�뵥��S��C2H5OH�������ͬһ�ⷿ�� | |
| C�� | ��װ�ò��Ͼ���ϩ��������ϩ�ȶ������� | |
| D�� | ��ɫ��ѧ�ĺ��ľ���������ҵ�����Ի�����������Ⱦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
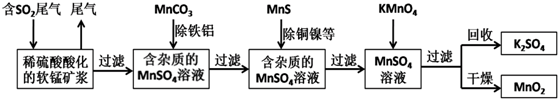
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | O2��O3 | B�� | CH3CH3��CH3CH2CH2CH3 | ||
| C�� | H��D | D�� | ��������춡�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
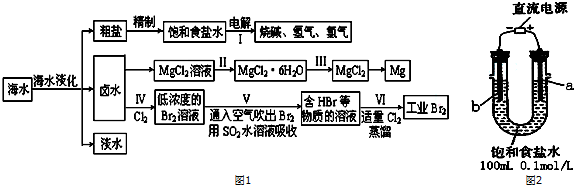
| ���� | Fe��OH��3 | Fe��OH��2 | Al��OH��3 | Mg��OH��2 |
| ��ʼ����pH | 2.7 | 8.1 | 3.8 | 9.5 |
| ��ȫ����pH | 3.7 | 9.6 | 4.8 | 11.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
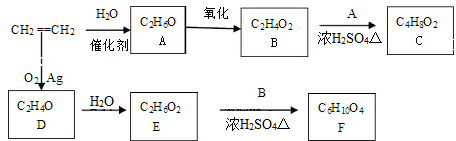
 CH3COOC2H5+H2O���÷�Ӧ������Ϊ������Ӧ����ȡ����Ӧ����
CH3COOC2H5+H2O���÷�Ӧ������Ϊ������Ӧ����ȡ����Ӧ����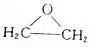 ��д��D��H2O��Ӧ����E�Ļ�ѧ����ʽ
��д��D��H2O��Ӧ����E�Ļ�ѧ����ʽ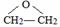 +H2O$\stackrel{һ������}{��}$HOCH2CH2OH��
+H2O$\stackrel{һ������}{��}$HOCH2CH2OH��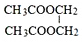 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ж������������������ͷ����� | |
| B�� | ��ɫ��K2Cr2O7����ˮ��Һ�����Ҵ�Ѹ����������ɫCr3+�������Ҵ��л�ԭ�� | |
| C�� | ����±���������Է�����ȥ��Ӧ��ˮ�ⷴӦ | |
| D�� | ��ʳ��ȥ��ˮ���е�ˮ��ʱ����������ˮ�ⷴӦ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com