
| 1mol |
| 1L |


 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��R�Ļ�ѧʽΪXmYnZp��X��Y��ZΪ���ֲ�ͬ���ӣ��������Ԫ�ؾ�Ϊ������Ԫ�أ�ȡ���ݵ�������Ũ��R��ˮ��Һ��5 mL��������ʵ�飺
��ȡһ��R��ˮ��Һ�������Ba(OH)2��Һ��Ӧ����Ӧ���������ɰ�ɫ�����������������Ӻ���٣�����ʣ�������2.33g���Ҽײ�����ϡ���ᡣ
��ȡ��һ��R��ˮ��Һ�������NaOH��Һ��ϼ��ȣ����ɾ��д̼�����ζ��������0.112L����״����������ʹʪ��ĺ�ɫʯ����ֽ������
�ݴ˻ش�
��1��д��R�ͼĻ�ѧʽ��R ���� ��
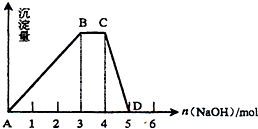
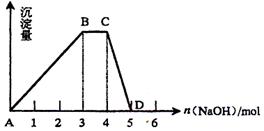 ��2����ȡ1L R��ˮ��Һ����������μ���NaOH��Һ�����ó���������NaOH�����ʵ�����ϵ��ͼ��ʾ����
��2����ȡ1L R��ˮ��Һ����������μ���NaOH��Һ�����ó���������NaOH�����ʵ�����ϵ��ͼ��ʾ����
��R��Һ�����ʵ���Ũ��Ϊ ��
��BC�α�ʾ�ĺ����� ��
��CD�η�Ӧ�����ӷ���ʽΪ ��
![]() ѧ����
ѧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��R�Ļ�ѧʽΪXmYnZp��X��Y��ZΪ���ֲ�ͬ���ӣ��������Ԫ�ؾ�Ϊ������Ԫ�أ�ȡ���ݵ�������Ũ��R��ˮ��Һ��������ʵ�飺
��ȡһ��R��ˮ��Һ�������Ba(OH)2��Һ��Ӧ����Ӧ���������ɰ�ɫ�����������������Ӻ���٣�����ʣ�������4.66g���Ҽײ�����ϡ���
��ȡ��һ��R��ˮ��Һ�������NaOH��Һ��ϼ��ȣ����ɾ��д̼�����ζ��������0.224L����״����������ʹʪ��ĺ�ɫʯ����ֽ�������ݴ˻ش�
��1��д��R�ͼĻ�ѧʽ��R ���� ��
��2����ȡ500mL R��ˮ��Һ����������μ���NaOH��Һ�����ó���������NaOH�����ʵ�����ϵ��ͼ��ʾ����
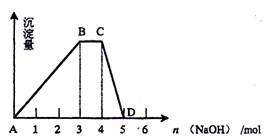
��B�㴦��Һ������Ũ�ȴӴ�С��˳���� ��
��R��Һ�����ʵ���Ũ��Ϊ ��
��BC�α�ʾ�ĺ����� ��
��CD�α�ʾ�ĺ����� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com