
 19.
19.
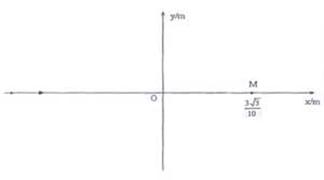
��ͼ��ʾ�����������x�������� ��̶�һ���ĵ��ɣ����� ���������ĵ糡�ֲ���y��Ϊ���ޡ���x�Ḻ����Զ��ԭ��ij����һ���ӷ���Դ������x�����������ٶ���ͬ�Ĵ���������ӡ��������� ������ ������ ��Ϊʹ�������ӽ���y���Ҳ��������Բ���˶������մ���λ��x����������Ҳ��ӫ�����ϣ�δ������������y������һ������ֱֽ�����⡢Բ����x���ϵ�Բ����ǿ�ų�������Ÿ�Ӧǿ�� �������������������������� ����
��1������Բ����ǿ�ų������Բ�����ꡣ
��2�����Ӵų�������뾶��
�⣺��1�������ӽ���糡��������Բ���˶��Ĺ���뾶Ϊr
![]()
![]() ��3�֣�
��3�֣�
�������֪��Ҫʹ���ӽ���糡ʱ������Բ���˶������������ӱ�����N�����糡����ͼ��ʾ������MN=r������糡ʱ�ٶȷ���ֱ��MN ��2�֣�
�ɼ��ι�ϵ�ɵ�ON=0.3��m�� ![]() �ų�Բ������Ϊ
�ų�Բ������Ϊ ��3�֣�
��3�֣�
��2���������ڴų��е��˶�����뾶ΪR
![]()
![]() ��2�֣�
��2�֣�
��ͼ��ʾ���ɼ��ι�ϵ�ɵ����Ӵų�����İ뾶![]() ��4�֣�
��4�֣�

�����뷴˼��
�˶����̵����ֻ����ĩ����״̬��ʵ��Ҫ��ѧ�����������Ƶ��ķ��������������˶����̡����⣬�������н�ų��е��˶��Ǹ��ѵ㣬Ҫ��ѧ�������ӵ��˶���Χ��֪��С�ų��İ뾶������ͼ�й켣�д�������


 ������������ϵ�д�
������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ʾ��ij������������һ�����͵��ɣ�BΪ��գ�A���ѳ���2molSO2��1molO2����һ�������·������淴Ӧ2SO2+O2?2SO3����H=-QkJ/mol����Q��0��һ��ʱ�������ѱ��־�ֹ��SO2�ķ�Ӧ����Ϊ��0������A��Ѹ�ٳ���2molSO2��1molO2���������ٴα��־�ֹʱ��SO2�ķ�Ӧ����Ϊ�����ڴ˹����У�����˵����ȷ���ǣ�������
��ͼ��ʾ��ij������������һ�����͵��ɣ�BΪ��գ�A���ѳ���2molSO2��1molO2����һ�������·������淴Ӧ2SO2+O2?2SO3����H=-QkJ/mol����Q��0��һ��ʱ�������ѱ��־�ֹ��SO2�ķ�Ӧ����Ϊ��0������A��Ѹ�ٳ���2molSO2��1molO2���������ٴα��־�ֹʱ��SO2�ķ�Ӧ����Ϊ�����ڴ˹����У�����˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
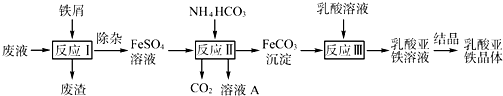
| c(Ti3+)2?c(Fe2+)2 |
| c(TiO2+)2?c(H+)4 |
| c(Ti3+)2?c(Fe2+)2 |
| c(TiO2+)2?c(H+)4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
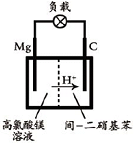 ��2012?����һģ��þ����Ͻ�㷺Ӧ���ں��պ��졢��ͨ����ص���ҵ������þ���Ʊ�������Ҫ�У�
��2012?����һģ��þ����Ͻ�㷺Ӧ���ں��պ��졢��ͨ����ص���ҵ������þ���Ʊ�������Ҫ�У�
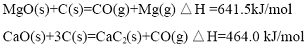
| n��CaC2��/N��MgO�� | ��ԭ�¶�/�� | ����ʱ��/h | ��ԭ��/% |
| 1.1 | 1110 | 2.0 | 62 |
| 1.1 | 1150 | 2.0 | 80 |
| 1.1 | 1150 | 2.5 | 85 |
| 1.2 | 1000 | 2.0 | 33 |
| 1.2 | 1150 | 2.0 | 84 |
| 1.2 | 1150 | 2.5 | 88 |
| 1.3 | 1150 | 2.0 | 86 |
| 1.3 | 1150 | 2.0 | 88 |
| 1100-1250�� |
| ��� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
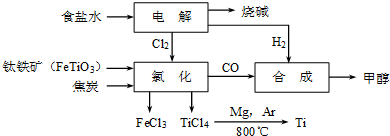
| TiCl4 | Mg | MgCl2 | Ti | |
| �۵�/�� | -25.0 | 648.8 | 714 | 1667 |
| �е�/�� | 136.4 | 1090 | 1412 | 3287 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com