
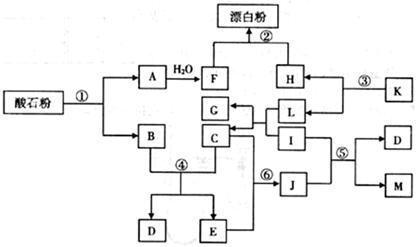
��ʯ����Ҫ�ɷ�ΪCaCO3������ͼ�����ʵ��ת���У�K��I��J���ճ������еĵ�ζ����I��J��M��GΪ�л��Mr(J)=60��Mr(M)=88����Ӧ�٢ڢ۾��ǹ�ҵ�����е���Ҫ��Ӧ��
��ش��������⣺
��1��K�Ļ�ѧʽ�� ��J�ķ���ʽ�� ��
��2����Ӧ���У���10g CaCO3����ʯ�ۣ���![]() �桢
�桢![]() kPaʱ����ȫ�ֽ�����A(s)��B(g)������a kJ���������ʲ���Ӧ�����÷�Ӧ���Ȼ�ѧ����ʽΪ ��
kPaʱ����ȫ�ֽ�����A(s)��B(g)������a kJ���������ʲ���Ӧ�����÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��3����Ӧ�ܵĻ�ѧƽ�ⳣ���ı���ʽ��K= ����֪![]() ��÷�Ӧ�� ��Ӧ������ȡ����ȡ�����
��÷�Ӧ�� ��Ӧ������ȡ����ȡ�����
��4����Ӧ�ݵĻ�ѧ����ʽΪ ��
��5����Ӧ������J��������ɫ��ѧԭ���ԭ���� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������⣺
��1���ó桢���ϵ����涣ҧ��ʱ��������ѪҺ��ע��һ�ֳ�֮Ϊ���ᣨ��Ҫ�ɷ�ΪHCOOH�����л��ᡣHCOOHͬCH3COOHһ����һ���л����ᡣд��HCOOH����Һ�е���ķ���ʽ��____________________________________��
��2�������ܵ��ó涣ҧʱ��Ƥ���ϳ���С�壬������Ϊ����ѪҺ�����ƽ�ⱻ�ƻ���������ҽ�����ƣ���һ��ʱ��С��Ҳ������Ȭ��������������ѪҺ���ָֻ���ԭ�ȵ����ƽ�⡣����ƽ���ƶ�ԭ������������ʵ__________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣���ʯ����Ҫ�ɷ�ΪCaCO3������ͼ�����ʵ��ת���У�K��I��J���ճ������еĵ�ζ����I��J��M��GΪ�л��Mr(J)=60��Mr(M)=88����Ӧ�٢ڢ۾��ǹ�ҵ�����е���Ҫ��Ӧ��
��ش��������⣺
��1��K�Ļ�ѧʽ�� ��J�ķ���ʽ�� ��
��2����Ӧ���У���10g CaCO3����ʯ�ۣ���![]() �桢
�桢![]() kPaʱ����ȫ�ֽ�����A(s)��B(g)������a kJ���������ʲ���Ӧ�����÷�Ӧ���Ȼ�ѧ����ʽΪ ��
kPaʱ����ȫ�ֽ�����A(s)��B(g)������a kJ���������ʲ���Ӧ�����÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��3����Ӧ�ܵĻ�ѧƽ�ⳣ���ı���ʽ��K= ����֪![]() ��÷�Ӧ�� ��Ӧ������ȡ����ȡ�����
��÷�Ӧ�� ��Ӧ������ȡ����ȡ�����
��4����Ӧ�ݵĻ�ѧ����ʽΪ ��
��5����Ӧ������J��������ɫ��ѧԭ���ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ʦ���С�����ʦ���С�����ʡ2010������ڶ������������ۣ���ѧ���� ���ͣ������
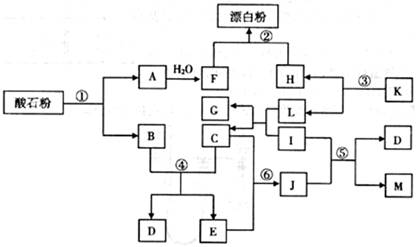
��14�֣���ʯ����Ҫ�ɷ�ΪCaCO3������ͼ�����ʵ��ת���У�K��I��J���ճ������еĵ�ζ����I��J��M��GΪ�л��Mr(J)=60��Mr(M)=88����Ӧ�٢ڢ۾��ǹ�ҵ�����е���Ҫ��Ӧ��
��ش��������⣺
��1��K�Ļ�ѧʽ�� ��J�ķ���ʽ�� ��
��2����Ӧ���У���10g CaCO3����ʯ�ۣ���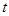 �桢
�桢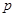 kPaʱ����ȫ�ֽ�����A(s)��B(g)������a kJ���������ʲ���Ӧ�����÷�Ӧ���Ȼ�ѧ����ʽΪ
��
kPaʱ����ȫ�ֽ�����A(s)��B(g)������a kJ���������ʲ���Ӧ�����÷�Ӧ���Ȼ�ѧ����ʽΪ
��
��3����Ӧ�ܵĻ�ѧƽ�ⳣ���ı���ʽ��K=
����֪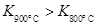 ��÷�Ӧ��
��Ӧ������ȡ����ȡ�����
��÷�Ӧ��
��Ӧ������ȡ����ȡ�����
��4����Ӧ�ݵĻ�ѧ����ʽΪ ��
��5����Ӧ������J��������ɫ��ѧԭ���ԭ���� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com