
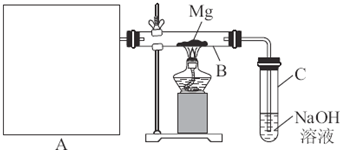
�������»�ѧʵ�鷽������Ƽ�Ҫ�����ʡ��Ʊ��ͼ���ͼ��ʾװ�ý���Mg��SO2��Ӧ��ʵ�顣

��1��ѡ����ȡSO2�ĺ����Լ�____________________________________________��
��10����H2SO4��Һ ��98����H2SO4��Һ ��Na2SO3���� ��CaSO3���� ��Cu
��2��д��װ��B�з�������Ҫ��Ӧ�Ļ�ѧ����ʽ___________________________________��
װ��C��NaOH��Һ��������_______________________________________________��
��3������Ϊ��װ���Ƿ��в���֮����________________________________������У���һһ˵��____________________________________________________________��
��ij�о�С������ˡ�ʵ������Si�����о��������Կα�Ϊ�������������ϵõ����¿ɹ��ο�����Ϣ��
�ٹ�ҵ���ڸ���ʱ��C��ԭSiO2���Ƶ�Si ��Mg�ڵ�ȼ�������¼�����SiO2��Ӧ �۽����軯����ϡH2SO4��Ӧ������������SiH4 ��Si��SiO2������ϡH2SO4��Ӧ ��SiH4�ڿ�������ȼ
�������о������м����š�����ѡ�ú��ʵ����������˵������³�ַ�Ӧ����������ϡ�����ܽ������Ȼ����ˡ�ϴ�ӡ������������������ϡ�����ܽ�������ʱ�������б������ͻ������Ҳֻ��Ԥ��ֵ��63�����ҡ���
��4����С�顰ʵ������Si���Ļ�ѧ����ʽ��_________________________________________��
��5������ơ���ϡ�����ܽ�������ʱ�������б������ͻ���ԭ����_________________
____________________________________________________________________��
(1) �ڢ�
(2)SO2+Mg![]() 2MgO+S ���ն����SO2����ֹSO2��Ⱦ����
2MgO+S ���ն����SO2����ֹSO2��Ⱦ����
(3)�� ��A��B֮��ȱ�ٸ���װ�ú�Cװ���������ͨ
(4)2Mg+SiO2![]() 2MgO+Si
2MgO+Si
(5)����þ�����ɵĹ������Ӧ�õ��軯þ���軯þ�����ᷴӦ����SiH4����ȼ
���û�ѧ����ʽ˵����
2Mg + Si====Mg2Si
Mg2Si + 2H2SO4====2MgSO4 + SiH4��
SiH4 + 2O2====SiO2 + 2H2O
��������.��1��������ȡSO2��A�����ȣ�����ȡSO2���Լ�ֻ����98%ŨH2SO4��Na2SO3���塣��2��SO2��Mg��Ӧ�Ļ�ѧ����ʽΪSO2+2Mg![]() 2MgO+S��C��NaOH��������β���е�SO2����ֹ����Ⱦ��������3�����װ�õIJ���֮���У�A��B��ȱ�ٸ���װ�ã�Cװ���������ͨ��
2MgO+S��C��NaOH��������β���е�SO2����ֹ����Ⱦ��������3�����װ�õIJ���֮���У�A��B��ȱ�ٸ���װ�ã�Cװ���������ͨ��
��.��4��SiO2+2Mg![]() 2MgO+Si����5����������Si��Mg��Ӧ���ɵ�Mg2Si����H2SO4�ܽ�����з�����Ӧ��Mg2Si+2H2SO4====2MgSO4+SiH4����SiH4����ȼ�����³��ֱ������ͻ�
2MgO+Si����5����������Si��Mg��Ӧ���ɵ�Mg2Si����H2SO4�ܽ�����з�����Ӧ��Mg2Si+2H2SO4====2MgSO4+SiH4����SiH4����ȼ�����³��ֱ������ͻ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
| ||
| ||


| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||

| ||
| ||
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�����о���ѧϰС��ֱ���ݲ�ͬ���Ϳ�������о����䷽�����£�

��.��һ���о���ѧϰС������ͼ��ʾ��װ�ý���þ���������Ӧ��ʵ�顣
(1)ʵ������ȡ����������������ȡ������̼��������������ѡ����ʵ��Լ���ȡ��������______________________��
A.10%������ B.80%������
C.Ũ���� D.ϡ����
E.�������ƹ��� F.������ƹ���
G.��������������Һ
(2)��Ӧ����B�ܱ��е���ɫ�����ĩ������ȡ��Ӧ��B�й�������ϡ�����в������г�������ζ�����壬д��B���з������йػ�ѧ��Ӧ����ʽ��______________________��
(3)����Ϊ��װ���Ƿ��в���֮����______________������У���д���Ľ�������______________________________��
��.�ڶ����о���ѧϰС������ˡ�ʵ�����ƹ衱���о��������Կα�Ϊ�������������ϵõ����¿ɹ��ο�����Ϣ���ٹ�ҵ���ڸ���ʱ��ľ̿�ۻ�ԭ����������Ƶù� ��þ�ڵ�ȼ�����¼�����������跴Ӧ �۽����軯����ϡ���ᷴӦ��������������� �ܹ�Ͷ������������ϡ���ᷴӦ �ݹ����ڿ���������ȼ
�������о������м����š�����ѡ�ú��ʵ����������˵������³�ַ�Ӧ����������ϡ�����ܽ������Ȼ����ˡ�ϴ�ӡ������������������ϡ�����ܽ�������ʱ�������б������ͻ������Ҳֻ��Ԥ��ֵ��63%���ҡ���
(4)��С�顰ʵ�����ƹ衱�Ļ�ѧ����ʽ��_________________________________________��
(5)����ơ���ϡ�����ܽ�������ʱ�������б������ͻ���ԭ����_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��.����ͼ��ʾװ�ý���Mg��SO2��Ӧ��ʵ�顣
(1)ѡ����ȡSO2�ĺ����Լ�_________________��
��10%��H2SO4��Һ ��80%��H2SO4��Һ ��Na2SO3���� ��CaSO3����
(2)д��װ��B�з�������Ҫ��Ӧ�Ļ�ѧ����ʽ__________________________ ��װ��C��NaOH��Һ��������__________________��
(3)����Ϊ��װ���Ƿ��в���֮����__________������У���һһ˵��__________________��
��.ij�о�С������ˡ�ʵ������Si�����о��������Կα�Ϊ�������������ϵõ����¿ɹ��ο�����Ϣ��
�ٹ�ҵ���ڸ���ʱ��C��ԭSiO2���Ƶ�Si��
��Mg�ڵ�ȼ�������¼�����SiO2��Ӧ��
�۽����軯����ϡH2SO4��Ӧ������������SiH4
��Si��SiO2������ϡH2SO4��Ӧ��
��SiH4�ڿ�������ȼ��
�������о������м����ţ�������ѡ�ú��ʵ����������˵������³�ַ�Ӧ����������ϡ�����ܽ������Ȼ����ˡ�ϴ�ӡ������������������ϡ�����ܽ�������ʱ�������б������ͻ������Ҳֻ��Ԥ��ֵ��63%���ҡ���
(4)��С�顰ʵ������Si���Ļ�ѧ����ʽ��___________________________��
(5)����ơ���ϡ�����ܽ�������ʱ�������б������ͻ���ԭ����___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������ʡ���������и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��11�֣���֪SiO2��SO2��CO2���������������ѧ���ʾ���һ���������ԣ�Mg��Na�Ļ�ѧ����Ҳ����һ���������ԡ�����ͼ��ʾװ�ý���Mg��SO2��Ӧ��ʵ�顣
��1��ѡ����ȡSO2�ĺ����Լ� ��
��10%��H2SO4��Һ ��80%��H2SO4��Һ
��Na2SO3���� ��CaSO3����
��2��д��װ���Һ�B�з�������Ҫ��Ӧ�Ļ�ѧ����ʽ��
��
��
װ��C��NaOH��Һ�������� ��
��3������Ϊ��װ���Ƿ��в���֮���� ������У���˵�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com