
|
��֪�л�������Aֻ��C��H����Ԫ���������ʹ��ˮ��ɫ�������������������һ�����ҵ�ʯ�ͻ�����չˮƽ��B��D�Ǽ�ͥ�еij������ʣ�A��B��C��D��E�����¹�ϵ��
�������ƶϲ���ȷ���� | |
| [����] | |
A�� |
����A�ͼ���������Ը��������Һ |
B�� |
D�к����Ȼ�������D���ʿ������ˮ���е�ˮ�� |
C�� |
����C�Ľṹ��ʽΪCH3CHO��E������Ϊ�������� |
D�� |
B��DE�Ļ�ѧ����ʽΪ�� |
 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
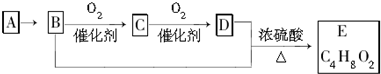
| A������A�ͼ����ѡ��ʹ�����Ը��������Һ | |||
| B��D�к��еĹ�����Ϊ�Ȼ�������D���ʿ������ˮ���е�ˮ�� | |||
| C������C�Ľṹ��ʽΪCH3CHO��E������Ϊ�������� | |||
D��B+D��E�Ļ�ѧ����ʽΪ��CH3CH2OH+CH3COOH
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ѧ���¿γ̡���ѧ2����(³�ư�) ���ͣ�013
|
��֪���л�������Aֻ��C��H����Ԫ���������ʹ������Ȼ�̼��Һ��ɫ�������������������һ������ʯ�ͻ�����չˮƽ��A��B��C��D��E�����¹�ϵ��
��֪B�Ǿ���������ζ��Һ�壬����ˮ������������ܣ��������ƶ�����ȷ���� | |
| [����] | |
A�� |
����A�ͼ����ѡ��ʹ�����Ը��������Һ |
B�� |
D�к��е���Ҫԭ����Ϊ�Ȼ�������D���ʿ������ˮ���е�ˮ�� |
C�� |
����C�Ľṹ��ʽΪCH3CHO��E������Ϊ�������� |
D�� |
B��D��E�Ļ�ѧ����ʽΪ��CH3CH2OH��CH3COOH��CH3COOC2H5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�츣��ʡ��һ��ѧ�ڵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪���л�������Aֻ��̼��������Ԫ���������ʹ��ˮ��ɫ�������������������һ������ʯ�ͻ�����չˮƽ��A��B��C��D��E�����¹�ϵ��

�������ƶϲ���ȷ���� �� ��
A������A�ͼ����ѡ��ʹ�����Ը��������Һ
B��B�к��еĹ�����Ϊ�ǻ���B���ʿ�����ȼ�Ϻ����ԭ�ϣ�Ҳ��������ɱ������
C������C�Ľṹ��ʽΪCH3CHO��E������Ϊ��������
D��B��D��E�Ļ�ѧ����ʽΪ��CH3CH2OH+CH3COOH��CH3COOC2H5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ����һ�и�һ��ѧ�ڵڶ����¿���ѧ�Ծ����������� ���ͣ���ѡ��
��֪���л�������Aֻ��̼��������Ԫ���������ʹ��ˮ��ɫ�������������������һ������ʯ�ͻ�����չˮƽ��A��B��C��D��E�����¹�ϵ��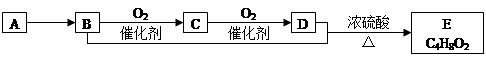
�������ƶϲ���ȷ���� �� ��
A������A�ͼ����ѡ��ʹ�����Ը��������Һ
B��B�к��еĹ�����Ϊ�ǻ���B���ʿ�����ȼ�Ϻ����ԭ�ϣ�Ҳ��������ɱ������
C������C�Ľṹ��ʽΪCH3CHO��E������Ϊ��������
D��B��D��E�Ļ�ѧ����ʽΪ��CH3CH2OH+CH3COOH��CH3COOC2H5
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com