
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)�γ����Ӽ������������Ӽ�ֻ���ھ���������(����)
(2)ȫ���ɷǽ���Ԫ���γɵĻ�����һ���ǹ��ۻ�����(����)
(3)ijЩ������ǽ���ԭ�Ӽ����γɹ��ۼ�(����)
(4)ijԪ�ص�ԭ�������ֻ��һ�����ӣ�����±�ؽ��ʱ�����γɵĻ�ѧ��һ�������Ӽ�(����)
(5)��ˮ��Һ���ܵ���Ļ�����һ�������ӻ�����(����)
(6)���ӻ��������κ�״̬�¶��ܵ���(����)
(7)�������Ӽ��Ļ������У�һ�������ӿ�ͬʱ�뼸���������γɾ�������(����)
 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±�ΪԪ�����ڱ���һ���֣���ش������й����⣺
| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | �� | ||
| 4 | �� | �� |
(1)�ݺ͢��Ԫ�ط�����________��________��
(2)��������õĽ�����________���ǽ�������ǿ��Ԫ����________(��дԪ�ط���)��
(3)�������γ��������������Ԫ����________���ֱ�д����Ԫ�ص�����������ޡ��������������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽ��________________________��______________________________��
(4)�����һ��ʵ�鷽�����ȽϢߡ��ⵥ�������Ե�ǿ����
___________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪������Τ�����в�������ø���Ƽ���������в������������Ի�����ϵķ�ʽ���еģ����и߶������ԣ�����ӽṹ������ʾ��

����˵����ȷ����(����)
A�������ʵķ���ʽΪC12H20N4O7
B��1 mol��������NaOH��Һ��Ӧ����������4 mol NaOH
C����һ�������£������ʿ��Է�����ȥ���ӳɡ�ȡ���ȷ�Ӧ
D����������ʹ��ˮ�����Ը��������Һ��ɫ������FeCl3��Һ������ɫ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�˵������ȷ���� (����)��
A����Ȼ������Ҫ�ɷ�ΪCH4
B��1 mol Cl2��1 mol CH4ǡ����ȫ��Ӧ����ת�Ƶĵ�������Ϊ2 NA
C����֪CH4��H2O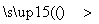 CH3OH��H2�÷�Ӧ���л�������������
CH3OH��H2�÷�Ӧ���л�������������
D��CH4�Ķ��ȴ���ֻ��һ�֣�����ʵ��˵��CH4Ϊ��������ṹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��.���и������ʣ�
��O2��O3����H2��D2��T2����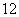 6C��
6C��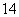 6C
6C
��CH3CH2CH2CH3��(CH3)2CHCH3
������Ͷ���
��CH3CH2CH2CH(C2H5)CH3��
CH3CH2CH2CH(CH3)C2H5
��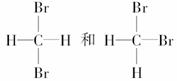
��Ϊͬ���칹�����________����Ϊͬλ�ص���______________________ __________________________________________________��
��Ϊͬ�����������________����ͬһ���ʵ���_______________________ _________________________________________________��
��.ij������A�ķ���ʽΪC5H11Cl���������ݱ����������к�������—CH3������CH2��һ��CH��һ��—Cl��
���Ŀ��ܽṹ�����֡���д�������ֿ��ܽṹ��ʽ��
(1)______________��(2)______________��
(3)______________��(4)______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)1 mol KHSO4�����ۻ��ɵ����2NA������(����)
(2)���ۻ���������ˮ�������ڹ��ۼ����ƻ�����������ˮ�������ڹ��ۼ������ƻ�(����)
(3)���ۻ������۵㶼�������ӻ�����(����)
(4)�����ڹ��ۼ�Խǿ������Խ�ȶ������ۡ��е�ҲԽ��(����)
(5)���������ӵĻ�����һ������������(����)
(6)�������Ӽ������ʲ������ǵ���(����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ȼ���е�CaF2�ֳ�өʯ����һ��������ˮ�Ĺ��壬���ڵ��͵����Ӿ��塣����һ����˵��CaF2�����Ӿ����ʵ����(����)
A��CaF2������ˮ����ˮ��Һ�ĵ����Լ���
B��CaF2���ۡ��е�ϸߣ�Ӳ�Ƚϴ�
C��CaF2���岻���磬��������״̬�¿��Ե���
D��CaF2���л��ܼ�(�籽)�е��ܽ�ȼ�С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪X��Y�������ӣ�Z�������ӣ�M��N�Ƿ��ӣ����Ƕ��ɶ�����Ԫ����ɣ��Ҿ������½ṹ���������ʣ���X��Y��M��N�ĺ������������ȣ��ڳ����£�M�Ǽ�������N�����壻��X��M������ͬ��Ԫ����ɣ���YΪ�������ӣ�����Z��ɵ����ʿ����ھ�ˮ����Z��ͬ����Ԫ����ɡ���ش��������⣺
(1)X�ĵ���ʽΪ______________________��Z�����ӷ���Ϊ______________________��
(2)X��Z�γɵĻ������������еĻ�ѧ������Ϊ______________________��
(3)д��Y��M��N�γɵĻ�������Ӧ�����ӷ���ʽ��
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ����С���о���������������ʱ���ı�ijһ������A2(g)��3B2(g)2AB3(g)��ѧƽ��״̬��Ӱ�죬�õ���ͼ��ʾ�ı仯����(ͼ��T��ʾ�¶�)(����)
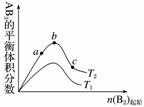
�ɴ˿ɵó��Ľ�����
A����Ӧ����a��b��c
B���ﵽƽ��ʱA2��ת���ʴ�СΪb��a��c
C����T2��T1��������Ӧһ���Ƿ��ȷ�Ӧ
D���ﵽƽ��ʱ��AB3�����ʵ�����СΪc��b��a
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com