���ڹ�����ռ����Ҫ�ĵ�λ�����������̽����
��1��������������ˮ����ͨ�����ȵ�̿������ˮú����C��s��+H
2O��g��?H
2��g��+CO��g����H=+131.3kJ/mol����S=+133.7J/K
�÷�Ӧ�ڵ������ܷ��Է�
����ܻ��
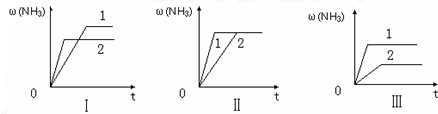
��2����֪��400��ʱ��N
2 ��g��+3H
2��g��?2NH
3��g����H��0 ��K
1=0.5��
��2NH
3��g��?N
2 ��g��+3H
2��g����K
2=
������ֵ����
��400��ʱ����0.5L�ķ�Ӧ�����н��кϳɰ���Ӧ��һ��ʱ����N
2��H
2��NH
3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��ӦV��N
2��
��
V��N
2��
�������������=������ȷ������
��3����֪��Ӧ��CO��g��+2H
2��g��?CH
3OH ��g����H��0
a���÷�Ӧ��һ�������´ﵽƽ����ڱ�֤H
2Ũ�Ȳ��������£�����������������Ը���ƽ�ⳣ�����ж�ƽ��
A��������Ӧ�����ƶ� B�����淴Ӧ�����ƶ� C�����ƶ�
b�����÷�Ӧ���淴Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ��
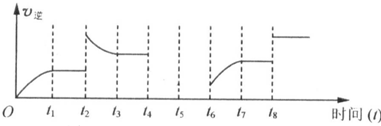
t
2ʱ��ƽ�ⳣ��K��t
1ʱ����ȿ���
������ĸ���ţ�
A������ B����С C�����䣮




 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�
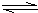 2NH3�ġ�H
2NH3�ġ�H