
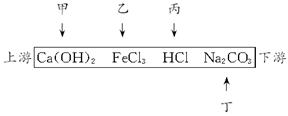
��һ����Ϻ����������С�ӱߣ������������������мס��ҡ������������������������ų�����ˮ�У�����ÿ��ֻ��̼���ơ����Ȼ������������ơ������е�һ�֡�ij��ѧ����С��Ժ�ˮ���ʱ���֣��ټ״�ˮ�����ɫ�����Ҵ�ˮ�ʺ��ɫ���۱���ˮ�ɻ���壻�ܶ����������ݣ���ˮ���塣�ش�
��1���ij���ˮ�зֱ�д��ѧʽ����___________����___________����___________����___________��
��2���ڶ���������M��ȡ��ˮ���У�һ����___________���ӡ�
��3����ˮ����ϺΪʲô�������棿
��1��Ca(OH)2 FeCl3 HCl Na2CO3
(2)H+��Na+��Ca2+��Fe3+��Cl-
��3����ˮ�еĻ�ѧ��Ⱦ��ֱ��Σ��ˮ����ֲ�������
��1���������⼰�й����������ͼ��
| ���� | ���� | ���� | ���� |
| �״� | ��ˮ�����ɫ | ��Ca��OH��2 | ֻ��Ca��OH��2�ܣ��������� |
| �Ҵ� | ��ˮ�ʺ��ɫ | �ҳ��ų�FeCl3 | ��Fe(OH)3���ɣ���Ca (OH)2��FeCl3 |
| ���� | ��ˮ�ɻ���� | �����ų�HCl | HCl�ܽ���Fe (OH)3��Ca��OH��2 |
| ���� | ��ˮ�壬������ | �����ų�Na2CO3 | ������HCl��Na2CO3��Ӧ����CO2 |
��2�����ڶ�����ˮ���壬�ʴ˴���ˮ�����ԣ��������������̼��������������µĸ����ӡ��������γɳ��������ۺϷ�����M����ˮ��Ӧ�ú���H+��Na+��Ca2+��Fe3+��Cl-�������Ậ����H+���õ�OH-��![]() �ȡ�
�ȡ�
��3����ˮ�еĻ�ѧ��Ⱦ��ֱ��Σ��ˮ����ֲ���������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��������и��⣺
��1�����������ų��ķ�Һ�ﺬ�е���Ⱦ���ǣ�
��_____________���ң�_____________����_____________����_____________�����ѧʽ��
��2���ڶ�������M��ȡ���ĺ�ˮ�У��϶����е�������______________��
��3��С������Ϻ����������ԭ����________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�ij���ˮ�зֱ�(д��ѧʽ)��___________����___________����___________����___________��
(2)�ڶ���������M��ȡ��ˮ���У�һ����___________���ӡ�
(3)��ˮ����ϺΪʲô�������棿_______________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�ij���ˮ�зֱ�(д��ѧʽ)��___________����___________����___________����___________��
(2)�ڶ���������M��ȡ��ˮ���У�һ����___________���ӡ�
(3)��ˮ����ϺΪʲô�������棿__________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ����Ϻ����������С�ӱߴ����ε������������������������ס��ҡ��������������ų��ķ�Һ�ÿ��ֻ����Na2CO3��FeCl3��Ca��OH��2��HCl�е�һ�֡�ij��ѧ����С��Ժ�ˮ���ʱ���֣��ټ״���ˮ�����ɫ�����Ҵ���ˮ�ʺ��ɫ���۱�����ˮ�ɻ���壻�ܶ����������ݣ���ˮ���塣

��������и��⣺
��1�����������ų��ķ�Һ�ﺬ�е���Ⱦ���ǣ�
��_____________���ң�_____________����_____________����_____________�����ѧʽ��
��2���ڶ�������M��ȡ���ĺ�ˮ�У��϶����е�������______________��
��3��С������Ϻ����������ԭ����____________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com