
 ��֪����������������ʯī�Ľṹ����ͼ��ʾ��������ѧ֪ʶ��ϸ�ͼ�ش��������⣮
��֪����������������ʯī�Ľṹ����ͼ��ʾ��������ѧ֪ʶ��ϸ�ͼ�ش��������⣮���� ��1������ͼ�ν�����������������㣬��������ԭ�ӣ���ÿһ���������������ι��ã��ݴ˼��㾧����N��Bԭ����Ŀȷ����ѧʽ��
��2����ͬ�ǽ���Ԫ��֮�����γɼ��Լ�������֮����ڷ��Ӽ���������
��3�������д��ڿ��������ƶ��ĵ����ܵ��磻
��4��ԭ�Ӿ����У������۵���ԭ�Ӱ뾶�������ɷ��ȣ�
��5��������������ṹ�ṹ�����ڽ��ʯ����������������ԭ�Ӿ��壻������������Nԭ�Ӵ���8�������6�����ģ���ԭ������Χ��4����ԭ���γ�������ṹ����ԭ������Χ��4����ԭ���γ�������ṹ���ݴ˷����жϾ����к��е�B��Nԭ�����Լ�Bԭ����Nԭ��֮�乲�ۼ�����λ������Ŀ�ȣ�
��� �⣺��1����������������������ʯī�Ľṹ��ͼʾΪ�������Σ�����֪ÿ������������ռ�е�ԭ����Ϊ6������ÿһ���㶼��3�������ι��õģ�������Nԭ����Ŀ=6��$\frac{1}{3}$=2��Bԭ����Ŀ=2���ʵ�����Ļ�ѧʽΪBN��
�ʴ�Ϊ��BN��
��2����ͬ�ǽ���Ԫ��֮�����γɼ��Լ�������B-Nԭ��֮����ڼ��Թ��ۼ�������֮����ڷ��Ӽ������������Բ�֮����ڷ��Ӽ���������
�ʴ�Ϊ�����Թ��ۼ������Ӽ���������
��3��������������ṹ���ṹ��û�����ɵ��ӣ����Բ����磬
�ʴ�Ϊ��������������ṹ���ṹ��û�����ɵ��ӣ�
��4��ԭ�Ӿ����У������۵���ԭ�Ӱ뾶�������ɷ��ȣ���ԭ�ӵİ뾶�ȵ�ԭ�Ӵ�N-B���ۼ�������B-PС�����ܴ����Ե���������۵�Ҫ��������ߣ�
�ʴ�Ϊ������͵���������ԭ�Ӿ��壬����ԭ�ӵİ뾶����ԭ��С��B-N���ۼ�������B-P���̣����ܴ����Ե���������۵�Ҫ��������ĸߣ�
��5��������������ṹ�ṹ�����ڽ��ʯ������ԭ�Ӿ��壻���ʯ�����������壬����8��������8��̼ԭ�ӣ�6�������6��̼ԭ�ӣ��������ڲ�����4��̼ԭ�ӣ���ͼ��ʾ��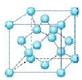 �����Խ��ʯ��һ�������к��е�̼ԭ����=8��$\frac{1}{8}$+6��$\frac{1}{2}$+4=8�������������е�ԭ������Χ��4����ԭ���γ�������ṹ����ԭ������Χ��4����ԭ���γ�������ṹ�����������������Ӧ�ú���4��N��4��Bԭ�ӣ�Bԭ���������3�����ӣ��γ�4�����ۼ�������1����λ������Bԭ����Nԭ��֮�乲�ۼ�����λ������Ŀ��Ϊ3��1��
�����Խ��ʯ��һ�������к��е�̼ԭ����=8��$\frac{1}{8}$+6��$\frac{1}{2}$+4=8�������������е�ԭ������Χ��4����ԭ���γ�������ṹ����ԭ������Χ��4����ԭ���γ�������ṹ�����������������Ӧ�ú���4��N��4��Bԭ�ӣ�Bԭ���������3�����ӣ��γ�4�����ۼ�������1����λ������Bԭ����Nԭ��֮�乲�ۼ�����λ������Ŀ��Ϊ3��1��
�ʴ�Ϊ��4��4��3��1��
���� ���⿼�����ʽṹ�����ʣ��漰����������Ļ�ѧʽ���㡢��ѧ�������������֪ʶ�㣬��Щ���Ǹ�Ƶ���㣬�ѵ��Ǿ����ܶȼ��㣬��Ŀ�Ѷ��еȣ�


 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | 1 L 0.1 mol•L-1 ��NH4��2Fe��SO4��2•6H2O��Һ�У�c��NH4+��+c��Fe2+��+c��H+��=c��OH-��+c��SO42-�� | |
| B�� | 0.1 mol•L-1��NH4Cl��0.1 mol•L-1��NH3•H2O�������ϣ�c��NH4+��+2c��H+��=c��NH3•H2O��+2c��OH-�� | |
| C�� | pH=9.4��Ũ�Ⱦ�Ϊ0.1 mol•L-1��HCN��NaCN�Ļ����Һ�У�c��Na+����c��CN-����c��HCN����c��OH-�� | |
| D�� | 0.1 mol•L-1 CH3COONa ��Һ��0.05 mol•L-1����������Ϻ��������Һ�У�c��CH3COO-����c��Cl-����c��CH3COOH����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����������һ�ֽྻ����������Դ��������������Ҫ�ɷ�ΪCO��CO2��H2�ȣ���H2��ϣ����ϳɼ״��������������õķ���֮һ��
����������һ�ֽྻ����������Դ��������������Ҫ�ɷ�ΪCO��CO2��H2�ȣ���H2��ϣ����ϳɼ״��������������õķ���֮һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ʵ����������ͼװ�ý����к��ȵIJⶨ����ش��������⣺
ʵ����������ͼװ�ý����к��ȵIJⶨ����ش��������⣺| ��Ӧ�� | ��ʼ�¶�t1/��C | �����¶�t2/��C | �к��� |
| HCl+NaOH | 13 | 19.8 | ��H1 |
| HCl+NH3•H2O | 13 | 19.3 | ��H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol H2O������Ϊ18 g/mol | |
| B�� | CH4��Ħ������Ϊ16 g/mol | |
| C�� | 22.4 L�κ���������ʵ�����Ϊ1 mol | |
| D�� | 1 mol �κ����ʾ�����NA������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��V1a=V2b��ϡ�ʹ˻����Һʱ����Һ�и�����Ũ��һ���������仯 | |
| B�� | ��pH��HA��+pH��BOH��=14����V1=V2ʱ�����ǡ����ȫ�к� | |
| C�� | �˻��Һ�У�2c��H+��+c��B+����c��OH-��+c��A-�� | |
| D�� | �������Һ��c��B+��=c��A-���������Һһ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ԫ����ǽ���Ԫ���γɵĻ�����һ�������ӻ����� | |
| B�� | ԭ������������Ϊ2��Ԫ��һ���������ڱ� IIA�� | |
| C�� | ���ӻ������в����ܴ��ڹ��ۼ� | |
| D�� | ����Ԫ��X��Y���γ�XY2�ͻ������X��Y��ԭ������֮�����Ϊ2��5 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com