
��ͼ��ijʵ��С������Ҵ���������װ�ã�
(1)ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ ��
��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�������Ӧ��________��Ӧ��
(2)��������ˮԡ���ò���ͬ����������____________________��
�ҵ�������______________________________________________��
(3)��Ӧ����һ��ʱ������Թ�a���ռ�����ͬ�����ʣ�������________������ƿ���ռ������������Ҫ�ɷ���________��
(4)���Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����________��
Ҫ��ȥ�����ʣ������ڻ��Һ�м���________(��д��ĸ)��
a���Ȼ�����Һ b���� c��̼��������Һ d�����Ȼ�̼
Ȼ����ͨ��________(��ʵ���������)���ɳ�ȥ��
��1��2Cu��O22CuO��CH3CH2OH��CuO
CH3CHO��Cu��H2O������
��2�����ȡ���ȴ ��3����ȩ���Ҵ���ˮ������ ��4�����ᡢc������
����:��1���ڼ��ȵ������£�ͭ���������ɺ�ɫ������ͭ��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ���������˵�����ɵ�����ͭ�ֱ���ԭ����������ͭ���ʣ�������ͭ���Ҵ���ԭ�����Ҵ���������������ȩ��Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵����Ӧһ��ˮ���ȷ�Ӧ��
��2����Ӧ����Ҫ�Ҵ������������Լ�ͨ�������������Ҵ����塣�Ҵ���������������ȩ����������Һ�壬����ͨ������ȴ�õ���ȩҺ�塣
��3�����ڷ�Ӧ���Ҵ����岻���ܱ���ȫ�������������Թ�a��һ������û�����ü���Ӧ���Ҵ�������a�е��������Ҵ�����ȩ��ˮ�������еĵ��������뷴Ӧ�����ͨ����ˮ���ռ�������ƿ�С�
��4������ֽ�Ժ�ɫ��˵����Һ�����ԣ���������ȩ���������������ᡣ����������ֻ��̼�����ƺ����ᷴӦ������ͨ��̼����������ȥ���ᣬ������ȩ��ˮ�ǻ��ܵģ�����Ӧͨ������õ���ȩ��


 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�� ���Ȼ�������ɫҺ�壬�е�Ϊ136�档������ˮ�⣬��������ˮ���������������̡���TiCl4+H2O=TiOCl2+2HCl��������650��~850���£�������ͨ���������Ѻ�̿�۵Ļ����ɵõ����Ȼ��Ѻ�һ���ж����塣��ͼ��ij����С���Ʊ�TiCl4�ķ�Ӧװ�ã�
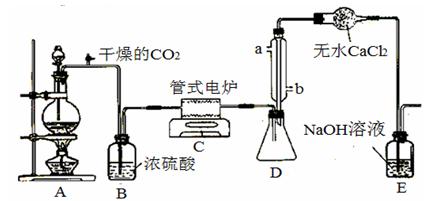
����Ҫ�����������£�
�����Ӻ�����װ�ã���ͨCl2ǰ��ͨ��CO2���岢����һ��ʱ�䣻
�ڵ���ƿ��TiCl4������������ʱ��ֹͣ���ȣ��Ӳ���и�ͨCO2����ֱ����¯�еĴɹ���ȴΪֹ��
�۽�TiO2��̿�ۻ�Ͼ��Ⱥ�װ���ʽ��¯�У�
�ܽ���¯���µ�800�棬һ��ʱ����ͨCl2��ͬʱ����������ͨ����ˮ��
�Իش��������⣺
��1����ȷ�IJ���˳��Ϊ������ţ� ;
��2��װ��A�еķ�Ӧ�����ӷ���ʽΪ ;
��3��Cװ���еķ�Ӧ�Ļ�ѧ����ʽΪ ��
�����ÿ���С�齫�����չ�����SO2��NaOH��Һע��װ��E������ʵ���β���������մ�����������β��һ��ʱ�������Һ��ǿ���ԣ��п϶�����Cl![]() ��CO32-��OH
��CO32-��OH![]() ��SO
��SO![]() �������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ������������ˮ�⣩.
�������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ������������ˮ�⣩.
��1������������裺
����1��ֻ����SO32-��
����2���Ȳ�����SO32-Ҳ������ClO-��
����3�� ��
��2�����ʵ�鷽��������ʵ�顣��д��ʵ�鲽���Լ�Ԥ������ͽ��ۡ���ѡʵ���Լ���
a��3mo l![]() L-1 H2SO4 b��0.01mol
L-1 H2SO4 b��0.01mol![]() L-1 KMnO4 c��1mol
L-1 KMnO4 c��1mol![]() L-1 BaCl2
L-1 BaCl2
d�����ۡ�KI��Һ e����̪�Լ� f��Ʒ����Һ
| ʵ�鲽�� |
|
| ����1��ȡ��������Һ���Թ��У��μ�3 mol | |
| ����2����A�Թ��еμ�1��2�� ������ţ��� | ����Һ �������1������ ���������2��3������ |
| ����3����B�Թ��еμ�1��2�� ������ţ��� | ����Һ �������3������ ����ϲ���2�������2������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ����е�һ��ѧ�߶����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��ͼ��ijʵ��С������Ҵ���������װ�ã�
(1)ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ ��
��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�������Ӧ��________��Ӧ��
(2)��������ˮԡ���ò���ͬ����������____________________��
�ҵ�������______________________________________________��
(3)��Ӧ����һ��ʱ������Թ�a���ռ�����ͬ�����ʣ�������________������ƿ���ռ������������Ҫ�ɷ���________��
(4)���Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����________��
Ҫ��ȥ�����ʣ������ڻ��Һ�м���________(��д��ĸ)��
a���Ȼ�����Һ b���� c��̼��������Һ d�����Ȼ�̼
Ȼ����ͨ��________(��ʵ���������)���ɳ�ȥ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ����и߶����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��ͼ��ijʵ��С������Ҵ���������װ�ã�
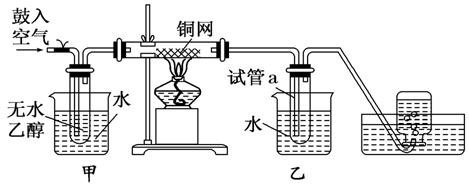
(1)ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ ��
��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�������Ӧ��________��Ӧ��
(2)��������ˮԡ���ò���ͬ����������____________________��
�ҵ�������______________________________________________��
(3)��Ӧ����һ��ʱ������Թ�a���ռ�����ͬ�����ʣ�������________������ƿ���ռ������������Ҫ�ɷ���________��
(4)���Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����________��
Ҫ��ȥ�����ʣ������ڻ��Һ�м���________(��д��ĸ)��
a���Ȼ�����Һ b���� c��̼��������Һ d�����Ȼ�̼
Ȼ����ͨ��________(��ʵ���������)���ɳ�ȥ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ�����п��Ի�ѧ�Ծ������� ���ͣ�ʵ����
��12�֣�ʵ���ҿ������Ҵ���ͭ��ͭ�Ļ������Ʊ���ȩ����ͼ��ij��ȤС����Ƶ�ʵ��װ�ã��ұߵķ�Ӧװ����ͬ������ߵ����巢��װ�ò�ͬ���Թ�C��װ��ˮ(����װ��δ����)���Իش�
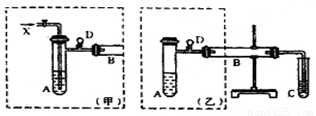
��1����������װ��ɺ�װ�Լ�ǰ����Ҫ���еIJ����� ��
��2������װ���е�A��B��������ȣ�A����ˮԡ���ȣ�B���� ���ȣ�A����ˮԡ���ȵ���Ҫ�ŵ��� ��
��3��������װ�ý���ʵ�飬B�ܴ�װͭ�ۣ���ͨ��A�ܵ�X�� ��B�з�Ӧ�Ļ�ѧ����ʽΪ ��
��4��������װ�ý���ʵ�飬��B����Ӧװ ��
B�з�Ӧ�Ļ�ѧ����ʽΪ ��
��5��ʵ�������C�Թ��е���Һ�������Ƶ�������ͭ��Һ�в����ȣ��ɹ۲쵽��
���� ��д���ù��̵Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com