
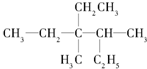
 ��ϵͳ������������3��4-����-3-�һ�����
��ϵͳ������������3��4-����-3-�һ�����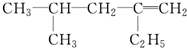
 �������б���̼ԭ����Ϊ4��������ͬһƽ���ϵ�̼ԭ�������Ϊ8
�������б���̼ԭ����Ϊ4��������ͬһƽ���ϵ�̼ԭ�������Ϊ8���� ��1��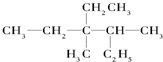 Ϊ�������̼������6��C������Ϊ���飬ѡȡ������������Ϊ��������Ŵ���߿�ʼ���ݴ�д�������ƣ�
Ϊ�������̼������6��C������Ϊ���飬ѡȡ������������Ϊ��������Ŵ���߿�ʼ���ݴ�д�������ƣ�
��2��4-��-2-��-��-1-��ϩ������Ϊ��ϩ��̼̼˫����1��C����4��C����1��������2��C����1���һ����ݴ�д����ṹ��ʽ��
��3�����������ĸ����ţ���ԭ�ӣ���̼ԭ��Ϊ����̼ԭ�ӣ��ڳ������л��������м�������������ṹ����ϩ�ͱ���ƽ���ͽṹ����Ȳ��ֱ���ͽṹ�������л�����ڴ˻����Ͻ��й��ߡ���������жϣ�ע�ⵥ��������ת��
��4���ṹ���ƣ�������������1���������ɸ�CH2���ŵĻ����ﻥ��Ϊͬϵ�ͬϵ���жϹ��ɣ���һ��������������ɸ�CH2ԭ���ţ�����Է����������һ�£�����Ȳ������еĹ�� ��һͬ��ͬͨʽ����һ�ƣ��ṹ���ƣ�
��5�����������ͨʽ����ʯ�����ص�����Ϊ������̼��ˮ������������m=nM��ʽ�����n���Ӷ��ó������ʽ������������к���3��Hд������ܵĽṹ��ʽ��
��� �⣺��1�� Ϊ�������̼������6��C������Ϊ���飬ѡȡ������������Ϊ��������3��4��C������1��������3��C����1���һ������л�������Ϊ��3��4-����-3-�һ����飬
Ϊ�������̼������6��C������Ϊ���飬ѡȡ������������Ϊ��������3��4��C������1��������3��C����1���һ������л�������Ϊ��3��4-����-3-�һ����飬
�ʴ�Ϊ��3��4-����-3-�һ����飻
��2��4-��-2-��-��-1-��ϩ�����л��������Ϊ��ϩ��̼̼˫����1��C����4��C����1��������2��C����1���һ�����ṹ��ʽΪ��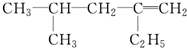
�ʴ�Ϊ��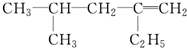 ��
��
��3�������ĸ����ţ���ԭ�ӣ���̼ԭ��Ϊ�������һ��е�̼ԭ�ӣ���������2���һ����ʷ����б���̼ԭ����Ϊ4�������д���C��C��ֱ�߽ṹ����C��C������C=C�е�̼ԭ�Ӵ�����Ȳ��Hԭ��λ�ã���ͬһֱ���ϵ�̼ԭ����Ϊ3�������д���C=C��ƽ���ͽṹ����C=Cֱ��������̼ԭ�Ӷ���ͬһƽ���ϣ�ͬʱC=C�е�1��̼ԭ����C��C�ϵ�2��̼ԭ�ӹ��ߣ����������ͬһƽ���ϵ�̼ԭ����Ϊ8��
�ʴ�Ϊ��4��8��
��4��ͬϵ���к��еĹ������������Ŀ������ͬ����CH2=CHCl�к���һ��̼̼˫�����������ʶ�û�У�����û����ڻ�Ϊͬϵ����л��
���к������������ӣ��������ʶ�����1�����߲�����ԭ�ӣ�����û����ܻ�Ϊͬϵ����л��
�ݺ͢�Ϊ���������ߺ���4��̼ԭ�ӣ�����ͬ���칹�壬������ͬϵ�
ֻ�Т�CH3CH2Cl�͢�CH3CH2CH2Cl ����ͬϵ�����������߷����ж�����1��Clԭ�ӣ�����֮�����1��CH2���ţ�
��ѡC��
��5����ʯ�����ص�Ϊˮ�Ͷ�����̼����������������ͨʽΪCnH2n+2��0.1mol��������ȫȼ������0.1nmol������̼��0.1��n+1��molˮ����44g/mol��0.1nmol+18g/mol��0.1��n+1��mol=39g����ã�n=6����������ķ���ʽΪ��C6H14Ϊ���飬������3����ԭ�ӣ���������ܵĽṹ��ʽΪ��CH3CH2CH2CH2CH2CH3��CH3C��CH3��2CH2CH3��
�ʴ�Ϊ��C6H14�� CH3CH2CH2CH2CH2CH3��CH3C��CH3��2CH2CH3��
���� ���⿼�����л���ṹ�����ʣ���Ŀ�Ѷ��еȣ���ȷ�����л���ṹ������Ϊ���ؼ���ע�������л�������ԭ��ͬ���칹�����дԭ������������ѧ�����Ӧ�û���֪ʶ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��50mL 1mol•L-1������Һ�е�������0.1mol•L-1Ba��OH��2��ҺAl3++2SO42-+2Ba2++3OH-�TAl��OH��3��+2BaSO4�� | |
| B�� | ��֪��ԭ��Fe2+��Br-��a mol FeBr2��Һ��ͨ��a mol Cl2��2Fe2++2Br-+2Cl2�TBr2+2Fe3++4Cl- | |
| C�� | ��KI��ϡ����Ļ����Һ��ͨ��������4H++O2+6I-�T3I2+2H2O | |
| D�� | �ڳ���ʯ��ˮ�м�������̼����þ��Һ��Ca2++OH-+HCO3-�TCaCO3��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���٢ڢ� | B�� | �ڢ� | C�� | �� | D�� | ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
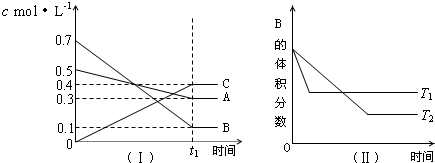
| A�� | �ڣ�t1+10��minʱ�����������������䣬����ѹǿ��ƽ�����淴Ӧ�����ƶ� | |
| B�� | ��t1+10��minʱ������������ѹǿ���䣬ͨ��ϡ�����壬ƽ�����淴Ӧ�����ƶ� | |
| C�� | �����������䣬�����¶ȣ������淴Ӧ���ʾ�������A��ת�������� | |
| D�� | ��ͼ����֪��T1��T2���Ҹ�����ӦΪ���ȷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͭƬ�ܽ⣬��������ȴ���ˮϡ����Һ����ɫ | |
| B�� | ���ɵ��������Ϊ1.12L | |
| C�� | �μӷ�Ӧ�������뱻��ԭ���������ʵ���֮��Ϊ2��1 | |
| D�� | ��ʵ���п���NaOH��Һ����β�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����״���µ�2.24L CO2ͨ��150mL 1mol•L-1 NaOH��Һ�У�������Һ��c��CO32-����c��HCO3-�� | |
| B�� | ������0.1 mol•L-1��������Һ��NH4Al��SO4��2 ��NH4Cl ��NH3•H2O��CH3COONH4�У�c��NH4+���ɴ�С��˳���ǣ��ڣ��٣��ܣ��� | |
| C�� | 0.1 mol•L-1 pHΪ9��NaHB��Һ�У�c��HB-����c��B2-����c��H2B�� | |
| D�� | �����£���0.4mol/L HA��Һ��0.2mol/LNaOH��Һ�������ϣ����Ի��ʱ��Һ����ı仯����û��ҺpH=5����c��A-��+c ��OH-����c��H+��+c��HA�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �٢� | C�� | �٢ڢ� | D�� | �٢ڢۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
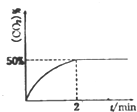 ������CO��SO2�̵�����Ⱦ��һ�ַ������ǽ����ڴ���������ת��Ϊ����S��g����������ӦΪ��2CO��g��+SO2��g��?S��g��+2CO2��g����
������CO��SO2�̵�����Ⱦ��һ�ַ������ǽ����ڴ���������ת��Ϊ����S��g����������ӦΪ��2CO��g��+SO2��g��?S��g��+2CO2��g����| T/�� | 200 | 300 | 400 |
| K | K1 | K2 | K3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HCl+AgNO3�TAgCl+HNO3 | B�� | MnO2+4HCl��Ũ���TMnCl2+Cl2��+H2O | ||
| C�� | 2HCl$\frac{\underline{\;ͨ��\;}}{\;}$H2��+Cl2�� | D�� | Mg+2HCl�TMgCl2+H2�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com