
| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Mg��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 11.2 |
| �¶�0C | 10 | 30 | 40 |
| CaSO4 | 0.19 | 0.21 | 0.21 |
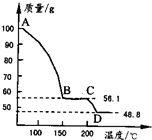
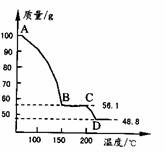
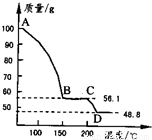
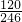 =48.8g��m��H2O��=100g-48.8g=51.2g��
=48.8g��m��H2O��=100g-48.8g=51.2g��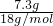 =0.41mol��n��MgSO4��=
=0.41mol��n��MgSO4��=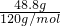 =0.41mol��
=0.41mol�� MgSO4+H2O����
MgSO4+H2O���� MgSO4+H2O����
MgSO4+H2O����

 �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Mg��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 11.2 |
| �¶�0C | 10 | 30 | 40 |
| CaSO4 | 0.19 | 0.21 | 0.21 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�����ڶ�ģ ���ͣ��ʴ���
| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Mg��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 11.2 |
| �¶�0C | 10 | 30 | 40 |
| CaSO4 | 0.19 | 0.21 | 0.21 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
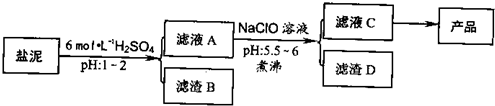
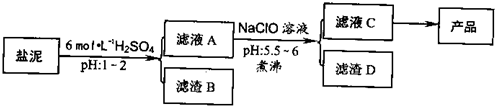
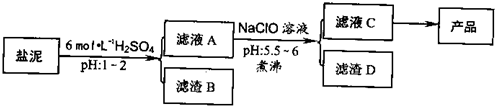
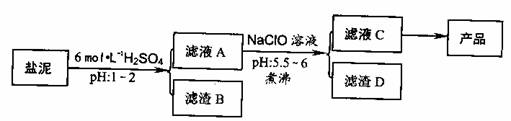
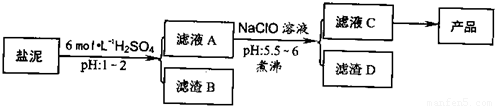
�ȼ���������ࣩ�к���þ�������Ĺ����κ�̼���Σ����к�þ����MgO�ƣ�Լ10%���ƣ���CaO�ƣ�Լ15%�����������ȵĺ�������1%���ȼ����������ȡMgSO4.7H2O���������£�
�����������������������ʱ��ҺpH
������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Mg(OH)2 |
pH | 5.2 | 3.2 | 9.7 | 11.2 |
�ܽ�ȱ�
�¶�0C | 10 | 30 | 40 |
CaSO4 | 0.19 | 0.21 | 0.21 |
�ش��������⣺
(1)����B����Ҫ�ɷ��ǣ�__________________
 (2)������A�õ���ҺC���ܷ��ð�ˮ����NaClO��_________,˵������________,���м�����е�Ŀ����_________________
(2)������A�õ���ҺC���ܷ��ð�ˮ����NaClO��_________,˵������________,���м�����е�Ŀ����_________________
(3)����ҺC�л�ò�Ʒ����3���������裬�ֱ���________��________,_______
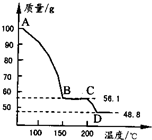
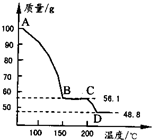
(4)��һ��������MgSO4.7H2O���������м��Ȳ�ò�ͬ�¶Ƚ�ʣ�������������ͼ��ʾ����ͼд��CD�η�Ӧ�Ļ�ѧ����ʽ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009��㶫ʡ�����и߿���ѧ��ģ�Ծ��������棩 ���ͣ������
| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Mg��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 11.2 |
| �¶�C | 10 | 30 | 40 |
| CaSO4 | 0.19 | 0.21 | 0.21 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com