
�������������ǽ�������Ʒ����ijЩ�����Ե���Һ�У��ڸ����ı����γ�һ�������������ļ������̡�����һ�ְ취�ǽ�������Ʒ�����������ƺ�Ũ�������ƵĻ����Һ�м��ȵ�130�淴Ӧ������̿��������»�ѧ����ʽ��ʾ��
��3Fe+NaNO2+5NaOH=3Na2FeO2+H2O+NH3��
��6Na2FeO2+NaNO2+5H2O=3 Na2FeO4+ NH3��+7NaOH
��Na2FeO2+ Na2FeO4+2H2O=Fe3O4+4NaOH
����˵����ȷ���� �� ��
A����Ӧ�ٲ���������ԭ��Ӧ
B�����������̲������κ���Ⱦ
C��������Ӧ�����У�ÿ��5.6gFe�μӷ�Ӧת��0.8mol����
D����Ӧ���е���������NaNO2
 ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2011?����ģ�⣩����ͭ������������ʹ�õĽ��������ǵĵ��ʼ�������Ӧ�÷dz��㷺��
��2011?����ģ�⣩����ͭ������������ʹ�õĽ��������ǵĵ��ʼ�������Ӧ�÷dz��㷺���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ��Ϫһ�и�һ��ѧ����ĩ������ѧ�Ծ����������� ���ͣ�ʵ����
��14�֣�ij�о�С�������������о���
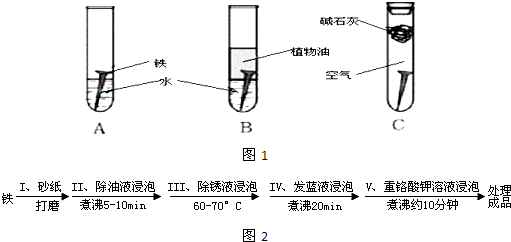
��1�������ϳ�ʱ���ͬѧ�۲쵽�������ǣ���ͼ�е������������������ ������ĸ����
��2������ʵ�������жϣ�����������ʴ�����У������ĵ缫��ӦΪ�� ��
��3����Ϊ�˷�ֹ�������⣬��С��ͬѧ���������������һ��������ý��������
A. �� B. ͭ C. п
��4����������ʴ����ҵ�����г���Ը������С�����������������Ч�������������ĸ�ʴ����ν���������������ڸ�������Ƚ�������������ʹ������γ�һ�����ܵ�����ɫ����Ĥ�������������̿ɱ�ʾ���£�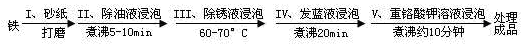
Ϊ���龭������������������Ƿ�ϸ�����Ʒ�������5%������ͭ��Һ�������Ʒ���ϸ�����������С�ɿף�δ�γ����ܵ�����Ĥ����һ��ʱ�佫�۲쵽������Ϊ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ��Ϫһ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��14�֣�ij�о�С�������������о���
��1�������ϳ�ʱ���ͬѧ�۲쵽�������ǣ���ͼ�е������������������ ������ĸ�����������Ҫ�ɷ���
��2������ʵ�������жϣ�����������ʴ�����У������ĵ缫��ӦΪ
��3����������ⲿ������ ���ڴ������£���������������ʴת��ΪFe��OH��2�ĵ�ط�Ӧ����ʽΪ
��4����Ϊ�˷�ֹ�������⣬��С��ͬѧ���������������һ��������ý��������
A. �� B. ͭ C. п
��5����������ʴ����ҵ�����г���Ը������С�����������������Ч�������������ĸ�ʴ����ν���������������ڸ�������Ƚ�������������ʹ������γ�һ�����ܵ�����ɫ����Ĥ�������������̿ɱ�ʾ���£�
�ٲ�����ó���Һ��15%��������Һ������������Ŀ�����ڳ�ȥ����������⣬�ò���Ӧ�����ӷ���ʽΪ___________________________________��
��Ϊ���龭������������������Ƿ�ϸ�����Ʒ�������5%������ͭ��Һ�������Ʒ���ϸ�����������С�ɿף�δ�γ����ܵ�����Ĥ����һ��ʱ�佫�۲쵽������Ϊ__________________________��
�۳����������ڷ���Һ��NaNO2��NaNO3��NaOH��ɵĻ��Һ���н��ݣ�����IV���������˸��ӵĻ�ѧ��Ӧ��
��Ӧһ��____Fe ��____NaNO2 ��___NaOH ��____Na2FeO2 ��____H2O ��___NH3��
��Ӧ����8Fe��3NaNO3 �� 5NaOH �� 2H2O �� 4Na2Fe2O4 �� 3 NH3��
��Ӧ����Na2FeO2 �� Na2Fe2O4 �� 2H2O �� Fe3O4 �� 4NaOH
��ƽ����Ӧһ���Ļ�ѧ����ʽ����ϵ��ֱ�����ں����ϣ�����Ҫѭ��ʹ�÷���Һ�������۽Ƕȷ�������Ҫ��ʹ�ù��ķ���Һ��_________
A. ֻ�����NaNO2 B. ֻ�����NaNO2��NaNO3
C. ��Ҫ����NaNO2��NaNO3��NaOH D. ��������κ����ʶ�ֱ��ʹ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��14�֣� ij�о�С�������������о���

��1�������ϳ�ʱ���ͬѧ�۲쵽�������ǣ���ͼ�е������������������ ������ĸ�����������Ҫ�ɷ���
��2������ʵ�������жϣ�����������ʴ�����У������ĵ缫��ӦΪ
��3����������ⲿ������ ���ڴ������£���������������ʴת��ΪFe��OH��2�ĵ�ط�Ӧ����ʽΪ
��4����Ϊ�˷�ֹ�������⣬��С��ͬѧ���������������һ��������ý��������
A. �� B. ͭ C. п
��5����������ʴ����ҵ�����г���Ը������С�����������������Ч�������������ĸ�ʴ����ν���������������ڸ�������Ƚ�������������ʹ������γ�һ�����ܵ�����ɫ����Ĥ�������������̿ɱ�ʾ���£�

�� ������ó���Һ��15%��������Һ������������Ŀ�����ڳ�ȥ����������⣬�ò���Ӧ�����ӷ���ʽΪ___________________________________��
�� Ϊ���龭������������������Ƿ�ϸ�����Ʒ�������5%������ͭ��Һ�������Ʒ���ϸ�����������С�ɿף�δ�γ����ܵ�����Ĥ����һ��ʱ�佫�۲쵽������Ϊ__________________________��
�� �����������ڷ���Һ��NaNO2��NaNO3��NaOH��ɵĻ��Һ���н��ݣ�����IV���������˸��ӵĻ�ѧ��Ӧ��
��Ӧһ��____Fe ��____NaNO2 ��___NaOH ��____Na2FeO2 ��____H2O ��___NH3��
��Ӧ����8Fe��3NaNO3 �� 5NaOH �� 2H2O �� 4Na2Fe2O4 �� 3 NH3��
��Ӧ����Na2FeO2 �� Na2Fe2O4 �� 2H2O �� Fe3O4 �� 4NaOH
��ƽ����Ӧһ���Ļ�ѧ����ʽ����ϵ��ֱ�����ں����ϣ�����Ҫѭ��ʹ�÷���Һ�������۽Ƕȷ�������Ҫ��ʹ�ù��ķ���Һ��_________
A. ֻ�����NaNO2 B. ֻ�����NaNO2��NaNO3
C. ��Ҫ����NaNO2��NaNO3��NaOH D. ��������κ����ʶ�ֱ��ʹ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com