
(16��)�����������壨FeSO4��7H2O����ҽҩ������Ѫ����ij����С������KMnO4��Һ�ζ��ķ������ⶨ�ò�Ѫ������Ԫ�صĺ���������������ʵ�飺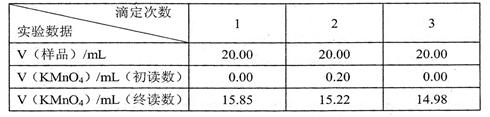
[��������]
�����������£���KMnO4����Һ����������ԭ�ζ������ԲⶨFe2+�ĺ�������Ӧ�����ӷ���ʽ�ǣ�Fe2++MnO4-+H+����Fe3++Mn2++H2O��δ��ƽ��
[��ʵ����Ʒ]
��������a.������ƽ��b.�ζ��ܣ�c.100mL��Ͳ��d.�ձ���e.©����f.����ƿ��100 mL�� 250mL����g.��ƿ��h.��������i.ҩ�ף�j.��ƿ��k.����̨�����ζ��ܼУ���l.��ͷ�ιܡ� [ʵ���¼]
[ʵ���¼]
[����������]
��1������ʵ����Ʒ�У�һ������Ҫ�������У�����ţ� ������Ҫ���Լ��У�����ţ� ��
�� 2����ʵ�����õ�KMnO4����Һ�����ʵ���Ũ��Ϊ
2����ʵ�����õ�KMnO4����Һ�����ʵ���Ũ��Ϊ  ��
��
��3������С������λͬѧ������������ֵζ���ʽ���гֲ�����ȥ����������ͬѧ�ǵ����ۣ����ȡ�ù�ʶ����Ϊ��������� ������ĸ��ţ���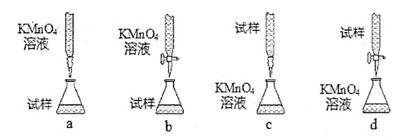
��4���жϵζ��յ�������� ��
��5�����ڽӽ��ζ��յ�ʱ������������ˮ����ƿ�ڱڳ� ϴһ�£��ټ����ζ����յ㣬������õIJ�Ѫ������Ԫ�صĺ��� ��ƫ��ƫС����Ӱ�죩��
ϴһ�£��ټ����ζ����յ㣬������õIJ�Ѫ������Ԫ�صĺ��� ��ƫ��ƫС����Ӱ�죩��
��6������ʵ�����ݣ�����ò�Ѫ������Ԫ�ص��������� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ������ѧ�ڵڶ����¿���ѧ�Ծ� ���ͣ�ʵ����
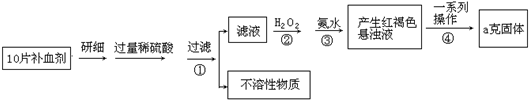
(8��)�����������壨 ����ҽҩ������Ѫ����ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�
����ҽҩ������Ѫ����ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�

(1) ����ڼ������H2O2��Ŀ�ģ�____________________
(2) ��ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص�����__________g(�ú�a�Ĵ���ʽ��ʾ)��
(3) ��С����Щͬѧ��Ϊ������.KMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ��
��д������KMnO4��Һ��FeSO4��Ӧ�����ӷ���ʽ______________________________
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ��������������ƽ�����������ձ�����ͷ�ι��⣬����____________________
������ʵ���е�KMnO4,��Һ��Ҫ�ữ�������ữ������__________��
a.ϡ���� b.ϡ���� c.ϡ���� d.Ũ����
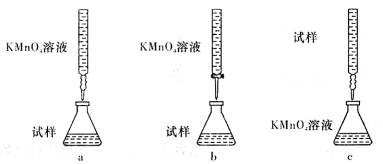
��ijͬѧ��Ƶ����еζ���ʽ��,���������__________ (�гֲ�����ȥ��(����ĸ��ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)�����������壨����ҽҩ������Ѫ����ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�
(1) ����ڼ������H2O2��Ŀ�ģ�____________________
(2) ��ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص�����__________g(�ú�a�Ĵ���ʽ��ʾ)��
(3) ��С����Щͬѧ��Ϊ������.KMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ��
��д������KMnO4��Һ��FeSO4��Ӧ�����ӷ���ʽ______________________________
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ��������������ƽ�����������ձ�����ͷ�ι��⣬����____________________
������ʵ���е�KMnO4,��Һ��Ҫ�ữ�������ữ������__________��
a.ϡ���� b.ϡ���� c.ϡ���� d.Ũ����
��ijͬѧ��Ƶ����еζ���ʽ��,���������__________ (�гֲ�����ȥ��(����ĸ��ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ������ѧ������ѧ�ڵڶ����¿���ѧ�Ծ� ���ͣ�ʵ����
(8��)�����������壨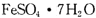 ����ҽҩ������Ѫ����
����ҽҩ������Ѫ���� ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�
ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�
(1) ����ڼ������H2O2��Ŀ�ģ�____________________
(2) ��ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص�����__________g(�ú�a�Ĵ���ʽ��ʾ)��
(3) ��С����Щͬѧ��Ϊ������.KMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ��
��д������KMnO4��Һ��FeSO4��Ӧ�����ӷ���ʽ______________ ________________
________________
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ��������������ƽ�����������ձ�����ͷ�ι��⣬����____________________
������ʵ���е�KMnO4,��Һ��Ҫ�ữ�������ữ������______ ____��
____��
a.ϡ���� b.ϡ���� c.ϡ���� d.Ũ����
��ijͬѧ��Ƶ����еζ���ʽ��,���������__________ (�гֲ�����ȥ��(����ĸ��ţ���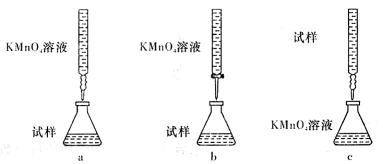
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com