
 ��
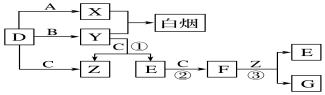
�� ���� �ǽ�������A�ܷ������������������յú�����DΪǿ�ᣬ��ѧ����͵�Ԫ�ػ��������ת����ϵ��
��1��A�ڳ�����Ϊ���壬B����ʹƷ����Һ��ɫ���д̼�����ζ����ɫ���壬��BΪSO2������ת����ϵ��֪AΪSԪ�ء�CΪSO3��DΪH2SO4��
��2����A�ڳ�����Ϊ��̬�������ʹʪ��ĺ�ɫʯ����ֽ��죬��AΪNH3��C�Ǻ���ɫ�����壬��CΪNO2�����ת����ϵ��֪BΪNO��DΪHNO3���ݴ˽��н��
��� �⣺�ǽ�������A�ܷ������������������յú�����DΪǿ�ᣬ��ѧ����͵�Ԫ�ػ��������ת����ϵ��
��1��A�ڳ�����Ϊ���壬B����ʹƷ����Һ��ɫ���д̼�����ζ����ɫ���壬��AΪSԪ�أ�BΪSO2��CΪSO3��DΪH2SO4��
�ٸ��ݷ���Ϣ��֪��DΪ���ᣬ�仯ѧʽΪ��H2SO4��
�ʴ�Ϊ��H2SO4��
���ڹ�ҵ������SO2����Ĵ����ŷű���ˮ���պ��γ����������Ⱦ�˻�����
�ʴ�Ϊ�����ꣻ
��B��C��Ӧ�Ƕ�������Ĵ�������Ӧ����Ӧ�Ļ�ѧ����ʽ��2SO2+O2$?_{��}^{����}$2SO3��
�ʴ�Ϊ��2SO2+O2$?_{��}^{����}$2SO3��
��2����A�ڳ�����Ϊ��̬�����C�Ǻ���ɫ�����壬��AӦΪNH3��BΪNO��CΪNO2��DΪHNO3��
�������Ϸ�����֪A��C�Ļ�ѧʽ�ֱ���NH3��NO2��
�ʴ�Ϊ��NH3��NO2��
�ڹ�ҵ���õ����������ڴ������������¸��¸�ѹ�ϳɰ�������Ӧ�Ļ�ѧ����ʽΪ��N2+3H2$?_{����}^{���¸�ѹ}$2NH3��
�ʴ�Ϊ��N2+3H2$?_{����}^{���¸�ѹ}$2NH3��
��AΪNH3��BΪNO����Ӧ����ʽΪ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O����Ӧ��OԪ�صõ��ӣ����ϼ۽��ͣ�NԪ��ʧ���ӣ����ϼ����ߣ�ת�Ƶ�����ĿΪ20������ת�Ƶķ������Ŀ�ɱ�ʾΪ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼��������ƶϣ���Ŀ�Ѷ��еȣ��������ʵ���ɫ�Լ���������������ӦΪͻ�ƿڽ����ƶϣ�ע���������ճ���Ԫ�ؼ��仯�������ʣ�����������ѧ���ķ���������������������


 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol A+2mol B+1mol C | |
| B�� | 0.6mol C+0.6mol D+0.2mol B+0.3mol A | |
| C�� | 0.6mol A+0.2mol D+0.1mol C | |
| D�� | 0.25mol A+0.5mol B+0.1mol C |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ${\;}_{23}^{51}$V��${\;}_{23}^{50}$V��Ϊͬλ�� | |
| B�� | ${\;}_{23}^{51}$��${\;}_{23}^{50}$V����������ͬ | |
| C�� | ${\;}_{23}^{51}$V��${\;}_{23}^{50}$V��ͬһ�ֺ��� | |
| D�� | ${\;}_{23}^{51}$V��${\;}_{23}^{50}$V�ĺ������������������Ϊ23 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ɰ�ɫ���������� | B�� | �����ɰ�ɫ���� | ||
| C�� | �������ݲ��� | D�� | �ޱ仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���� | ��; |
| A | �ƺͼصĺϽ��ܵ��� | ԭ�ӷ�Ӧ�ѵĵ��ȼ� |
| B | �����������Ư���� | Ư��ֽ�� |
| C | þȼ��ʱ����ҫ�۵�ǿ�� | ���������� |
| D | Al��OH��3�����ֽ� | ���ϵ���ȼ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ��һ��10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
ѧϰ���о���ѧ������Ҫ����ʵ�顣���й���ʵ�鰲ȫ��˵����ȷ���ǣ� ��

A. ��ȼ�ŵľƾ���ȥ��ȼ��һֻ�ƾ���
B. ��ˮ������ʵ��ʱ��Ҫ����ƿ��Ӽ�����ʯ�Է�ֹ����
C. ������Ũ��������Ƥ����Ҫ����������������Һ��ϴ
D. ʢ��������Լ�ƿ��Ҫ������ͼ�ı�־
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�߶���10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ���¶��µĶ��������У���������Щ���������ٷ����仯ʱ��������ӦA(g)��2B(g) C(g)��D(g)�Ѵﵽƽ��״̬���ǣ� ��
C(g)��D(g)�Ѵﵽƽ��״̬���ǣ� ��
�ٻ�������ѹǿ �ڻ��������ܶ� ��B�����ʵ���Ũ�� �ܻ������������ʵ��� �ݻ�������ƽ����Է������� ��v(C)��v(D)�ı�ֵ ��������������
A���ڢۢܢݢޢ� B���٢ۢܢ� C���٢ڢۢܢݢ� D���٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�߶��Ͻ�ѧ�ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
��Χ�����Ų�Ϊ3s23p5��Ԫ�������ڱ��е�λ����
A. �������ڢ�A��p�� B. �������ڢ�B��p��
C. �������ڢ�A��s�� D. �������ڢ�B��s��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com