
| ������ | H+��Na+��Al3+��Ag+��Ba2+ |
| ������ | OH-��Cl-��CO32-��NO3-��SO42- |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
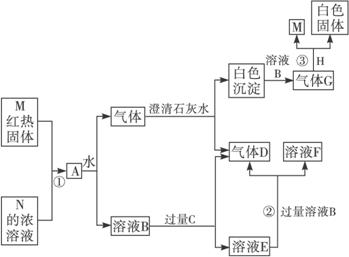
��1��д��C��D��H�Ļ�ѧʽ___________��___________��___________��
��2��д����Ӧ�٢ڢ۵Ļ�ѧ����ʽ��
��_______________________________________________,
��_______________________________________________��
��_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������A��B��C��D��E��F��G��H��M��N������C��HΪ�����������һ������A�ڳ����²�������Ӵ�ʱ�ܷ�����ͼ��ʾ�ı仯�������йط�Ӧ��������ȥ����������������⣺

��1��д��C��D��H�Ļ�ѧʽ___________��___________��___________��
��2��д����Ӧ�٢ڢ۵Ļ�ѧ����ʽ��
��_______________________________________________,
��_______________________________________________��
��_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�ʵ��������Ũ������MnO2��ȡCl2������Cl2��Ca(OH)2��Ӧ������Ư�ۡ���֪Cl2��Ca(OH)2��Ư���Ƿ��ȷ�Ӧ���¶��Ը���������Ӧ��
6Cl2��6Ca(OH)2��Ca(ClO3)2��5CaCl2��6H2O��������λͬѧ�ֱ���Ƶ�����ʵ��װ�����£�U�ι��з��в�����ά��������˳��ͨ������
��1���������������������ס��ҡ�������װ�õ���ȱ��������ۣ����ʵ���ѡ����������±��ڣ�
a�������Ʒ�Ӧ���� b�������Ʒ�Ӧ���� c���и���Ӧ����
d���ɷ�ֹ����Ӧ���� e��������Ⱦ���� f���ɷ�ֹ��Ⱦ����
|
| �ŵ� | ȱ�� |
| ��װ�� |
|
|
| ��װ�� |
|
|
| ��װ�� |
|
|
��2����Ӽס��ҡ�������װ���У�ѡȡ��������ɲ��֣�A��B��C��D��E��F��G������װһ������Ϊ�����Ƶ�ʵ��װ�ã�����˳�����������ҵķ���Ϊ ���ڴ�װ�ü��ס��ҡ�����װ���У�����Ϊ�Ƿ�ȱ�ٱ�Ҫ��װ�ã���˵��ԭ��
��
��3�������Ӧǰ������m g Ca(OH)2����Ӧ��������ȴ���������ʳ���Ϊn g���跴Ӧ�в�����ˮ�����ڹ��������У�����ù���������Ca(ClO)2�����������ı���ʽΪ�����ػ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���Ĵ�ʡ��֦���и�����һ��ͳ����ѧ�Ծ��������棩 ���ͣ�ѡ����
����E��F��G��M��N���ֿ��ܵ�ǿ����ʣ�������ˮ�е�������������ӣ��������Ӳ��ظ�����
|
������ |
H+��Na+��Al3+��Ag+��Ba2+ |
|
������ |
OH-��Cl-��CO32-��NO3-��SO42- |
��֪��
��E��F����Һ�ʼ��ԣ�G��M��N ��Һ�����ԡ�
����N��Һ����εμ�F��Һ�������������������Ӻ���ٵ�����ʧ��
��M��Һ������������Һ��Ӧ���ܲ���������
����˵����ȷ����
A��N��Һ�������F��Һ��Ӧ�����ӷ���ʽ�� Ba2++SO42-=BaSO4��
B��E��Һ��N��Һ��Ϸ�����Ӧ��2Al3++3CO32-+3H2O=2Al(OH)3��+3CO2��
C��M��Һ��F��Һ��ϲ����ij��������ܽ��ڹ�����ˮ��
D����G��Һ��μ��������������ʵ�����Ũ�ȵ�E��Һ�У���Ӧ�����ӷ���ʽΪ
2H++CO32-=CO2��+H2O
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com