
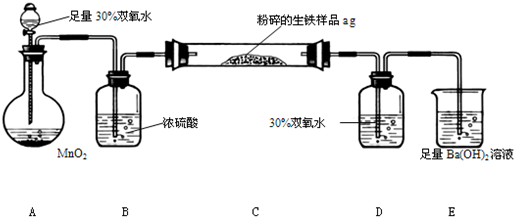
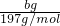 ��12g/mol=
��12g/mol=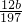 g��������̼����������Ϊ
g��������̼����������Ϊ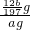 ��100%=
��100%=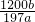 %��
%�� %��
%��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| 1200b |
| 197a |
| 1200b |
| 197a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�긣��ʡ��������У������ѧ�ڵ�һ��������ѧ�Ծ��������棩 ���ͣ�ʵ����
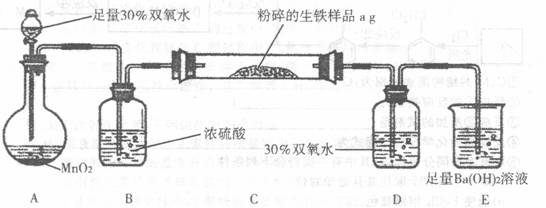
�����г����⣬����������Ԫ�أ���̼Ԫ�غ���Ԫ�ء�����̼��Ҫ��̼��������̬���ڣ���ʹ�������ܼ�Ӳ���࣬������������;����һ���������ֵ�ԭ�ϡ�ij��ȤС����ư���ͼ��ʾ��ʵ��װ�ã��ⶨ�����еĺ�̼����

A??????????? B????????????????? C????????????????? D???????? E
��ش��������⣺
��1������������������Ԫ�أ���ʹ���������ȴ��ԡ���Ԫ�������������п��ܴ��ڵļ�̬��???????????
A����2?? ����? B��0? �� ��?? C��+4?? ����?? D��+6
��2��д�����ձ�E�з�����Ӧ�����ӷ���ʽ��???????????????????????????? ��
��3��D��30% ˫��ˮ��������???????????????????????????????????????????????? ������װ�ã����ⶨ����̼����??????? ������ƫ��������ƫ����������Ӱ������
��4����Ӧ��ɺ�����֤����������Ԫ�أ�������Ƶ�ʵ�鷽���ǣ�д��ʵ�鲽�衢����???????????????????????????????????????????????????? ��
��5����C�ܵ���Ʒ��ַ�Ӧ�����E�����ɵij���Ϊbg���������������ĺ�̼��Ϊ???????????? ��
��6��ʵ������У�����ȤС��Ӧע��İ�ȫ������???????????????????? ������дһ�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
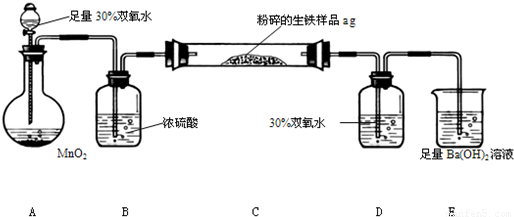
�����г����⣬������̼���������Ԫ�أ�����̼Ԫ����Ҫ��̼��������̬���ڣ���ʹ�������ܼ�Ӳ���࣬������������;����һ���������ֵ�ԭ�ϡ�ij��ȤС����ư���ͼ��ʾ��ʵ��װ��(C�ļ���װ��ʡ��)���ⶨ�����е�̼������������
��ش��������⣺
(1)�������������к�Ԫ�أ���ʹ���������ȴ��ԡ���Ԫ�������������п��ܴ��ڵļ�̬��___________________
A��-2 B��0 C��+4 D��+6
(2)д�����ձ�E�з�����Ӧ�����ӷ���ʽ��_________________________ ��
(3)D��30��˫��ˮ��������_________________________________ ��
����װ�ã����ⶨ��̼������������__________ (�ƫ�ߡ�����ƫ�͡���Ӱ�족)��
(4)��Ӧ��ɺ�����֤����������Ԫ�أ�������Ƶ�ʵ�鷽����(д��ʵ�鲽�衢����)
__________________________________________________________________
(5)��C�ܵ���Ʒ��ַ�Ӧ���E�����ɵij���Ϊbg��������������̼����������Ϊ_____________________��
(6)ʵ������У�����ȤС��Ӧע��İ�ȫ������______________��(��дһ��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�������������и������£��ڰ˴��¿���ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com