
| ���� | �� |
| 100��60% |
| 12 |
| 100��8% |
| 1 |
| 100��32% |
| 16 |
| 100��60% |
| 12 |
| 100��8% |
| 1 |
| 100��32% |
| 16 |
| NaOH |
| �� |
| NaOH |
| �� |
| 100��60% |
| 12 |
| 100��8% |
| 1 |
| 100��32% |
| 16 |
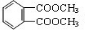 ����Ӧ����DAP����Ļ�ѧ����ʽ��
����Ӧ����DAP����Ļ�ѧ����ʽ�� ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ���� |

| NaOH |
| �� |
| NaOH |
| �� |
| NaOH��Һ | ������Һ | ����Cu��OH��2 | ������ | |
| X | �кͷ�Ӧ | ������ | �� �� | �������� |
| Y | ������ | ������ | ���Ⱥ��к�ɫ���� | �������� |
| Z | ˮ�ⷴӦ | ������ | ���Ⱥ��к�ɫ���� | ������ |
| W | ˮ�ⷴӦ | ������ | ������ | ������ |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
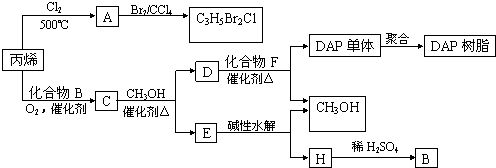
��ϩ�����ںϳ�ɱ�������߳��ũҩ(����ʽΪC3H5Br2Cl)��Ӧ�ù㷺��DAP��֬��

��֪���봼�ɷ���������������Ӧ��
RCOOR�䣫R��OH![]() RCOOR�士R��OH(R��R�䡢R���������)
RCOOR�士R��OH(R��R�䡢R���������)
(1)ũҩC3H5Br2Cl������ÿ��̼ԭ���Ͼ�����±ԭ�ӡ�
��A�Ľṹ��ʽ��_________________________________________________________��
A���еĹ�����������________________��
���ɱ�ϩ����A�ķ�Ӧ������______________��
(2)Aˮ��ɵõ�D����ˮ�ⷴӦ�Ļ�ѧ����ʽ��_____________________________��
(3)C�����ܶ�����ͬ״̬�¼����ܶȵ�6.25����C�и�Ԫ�ص����������ֱ�Ϊ��̼60%��
��8%����32%��C�Ľṹ��ʽ��________________��
(4)����˵����ȷ����(ѡ�������ĸ)________��
a��C�ܷ����ۺϷ�Ӧ����ԭ��Ӧ��������Ӧ
b��C������������������ͬ���칹����4��
c��D������IJ�����B������ͬ����Է�������
d��E���з�����ζ���������Ҵ�
(5)E��ˮ����ᆳ�������յõ��״���B�����߾���ѭ��������DAP��֬���Ʊ�����
�н��״���H����IJ���������________��
(6)F�ķ���ʽΪC10H10O4��DAP����Ϊ���Ķ�Ԫȡ���������ȡ���������ڶ�λ���õ��屽���ϵ�һ��ȡ����ֻ�����֡�D��F��Ӧ����DAP����Ļ�ѧ����ʽ��________________________________________________________________________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��������Ͽ��������ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ������
��ϩ�����ںϳ�ɱ�������߳��ũҩ������ʽΪC3H5Br2Cl����Ӧ�ù㷺��DAP��֬��

��֪���봼�ɷ���������������Ӧ��

��1��ũҩC3H5Br2Cl������ÿ��̼ԭ���Ͼ�����±ԭ�ӡ�
A�Ľṹ��ʽ��__________________��A�����������ŵ�������____________________��
��ϩ��A�ķ�Ӧ������_______________��A��C3H5Br2CI�ķ�Ӧ������_____________��
��2��Aˮ��ɵõ�D����ˮ�ⷴӦ�Ļ�ѧ����ʽΪ��
_______________________________________________________________________.
��3��C�����ܶ�����ͬ״̬�¼����ܶȵ�6.25����S�и�Ԫ�ص����������ֱ�Ϊ��̼60%����8%����32%. S�Ľṹ��ʽΪ_________________________________��
��4������˵����ȷ����______________������ĸ���ţ���
a��C�ܷ����ۺϷ�Ӧ����ԭ��Ӧ��������Ӧ
b��C����2������������ͬ���칹����4��
c��D������IJ�����B������ͬ����Է�������
d��E���з�����ζ���������Ҵ�
��5��E��ˮ����ᆳ�������յõ��״���B�����߾���ѭ��������DAP��֬���Ʊ������н��״���H����IJ���������__________________��
��6��F�ķ���ʽΪC10H10O4. ��DAP����Ϊ���Ķ�Ԫȡ���������ȡ���������ڶ�λ���õ��屽���ϵ�һ��ȡ����ֻ�����֡�����D��F��Ӧ����DAP����Ļ�ѧ����ʽΪ��
_______________________________________________________________________.
��7��ʵ������2-�����Ʊ���ϩʱ������������SO2�� CO2 ��ˮ������ijͬѧ�������Լ�
�������������壬�������ͨ���Լ���˳����_______________������ţ���
�ٱ���Na2SO3��Һ������KMnO4��Һ ��ʯ��ˮ ����ˮCuSO4��Ʒ����Һ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com