
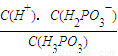 ��
��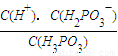 =
=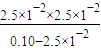 mol/L=8.3×10-3mol/L���ʴ�Ϊ8.3×10-3mol/L��
mol/L=8.3×10-3mol/L���ʴ�Ϊ8.3×10-3mol/L��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?����һģ���Ǒz�ᣨH3PO3���Ƕ�Ԫ�ᣬ������NaOH��Һ��Ӧ����Na2HPO3��
��2013?����һģ���Ǒz�ᣨH3PO3���Ƕ�Ԫ�ᣬ������NaOH��Һ��Ӧ����Na2HPO3���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ�����б�ҵ���ۺϲ��ԣ�һ�����ۻ�ѧ�Ծ��������棩 ���ͣ�������
�Ǒz�ᣨH3PO3)�Ƕ�Ԫ�ᣬ������NaOH��Һ��Ӧ����Na2HPO3��
��1��PCl3ˮ�����ȡ�����PCl3+3H2O=H3PO3+_______��
��2��H3PO3��Һ�д��ڵ���ƽ�⣺H3PO3 H++H2PO3����
H++H2PO3����
��ij�¶��£�0.10mol•L��1 �� H3PO3 ��Һ pH =1.6������Һ�� c(H+) =2.5��10��2 mol•L��1������¶�����������ƽ���ƽ�ⳣ��K��д��������̡���H3PO3�ĵڶ���������Բ��ƣ����������λ��Ч���֡���
�ڸ���H3PO3�����ʿ��Ʋ�Na2HPO3ϡ��Һ��pH________7 (�>������=����<������
��3�����������ǿ��ԭ�ԣ���ʹ��ˮ��ɫ���÷�Ӧ�Ļ�ѧ����ʽΪ_______��
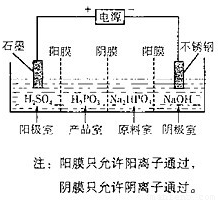
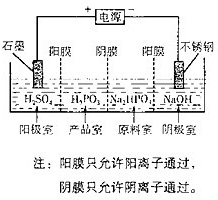
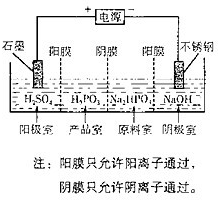
��4�����Na2HPO3��ҺҲ�ɵõ����[�ᣬװ��ʾ��ͼ���£�

�������ĵ缫��ӦʽΪ____________________________��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������һģ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com