
���� ��1������n=$\frac{m}{M}$������������ʵ�������ԭ�����ʵ���Ϊ�����2��������N=nNA������ԭ����Ŀ��
��2�����ݻ�ѧʽ��֪Na2R��n��Na+��=2n��Na2R�����ݴ˼��㺬��0.4molNa+��Na2R�����ʵ���������M=$\frac{m}{n}$����Na2R��Ħ��������
����n=$\frac{m}{M}$����1.6gR�����ʵ��������ݻ�ѧʽ��֪Na2R��n��Na2R��=n��R����
��3������������n=$\frac{V}{{V}_{m}}$=$\frac{m}{M}$���з���ʽ������⣻
��4������n=$\frac{V}{{V}_{m}}$������������ʵ���������M=$\frac{m}{M}$������Ħ����������g/molΪ��λʱ����Է���������ֵ�ϵ�����Ħ��������
��5������Na��Mg��Al��Ԫ�ص������ֱ�Ϊ23g��16g��9g������n=$\frac{m}{M}$����������ʵ���������ȷ��NaCl��MgCl2��AlCl3���ʵ���֮�ȣ�����n��Cl-��=n��NaCl��+2n��MgCl2��+3n��AlCl3������������ʵ������ٸ���m=nM����������������
��� �⣺��1��147g H2SO4�����ʵ�����$\frac{147g}{98g/mol}$=1.5mol�����к�����ԭ�����ʵ���Ϊ1.5mol��2=3mol��������ԭ����Ϊ3mol��4��6.02��1023mol-1=1.806��1024
���ʴ�Ϊ��1.5 mol��1.806��1024����
��2�����ݻ�ѧʽ��֪Na2R��n��Na+��=2n��Na2R�������Ժ���0.4molNa+��Na2R�����ʵ���Ϊ$\frac{0.4mol}{2}$=0.2mol������Na2R��Ħ������Ϊ$\frac{12.4g}{0.2mol}$=62g/mol��
��Ħ��������g/mol����λ����ֵ�ϵ�������Է�����������Na2R����Է�������Ϊ62������R�����ԭ������Ϊ62-23��2=16��
1.6gR�����ʵ���Ϊ$\frac{1.6g}{16g/mol}$=0.1mol�����ݻ�ѧʽ��֪Na2R��n��Na2R��=n��R��=0.1mol��
�ʴ�Ϊ��62g/mol��0.1mol��
��3��������������ʵ���֮��Ϊ$\frac{39.2L}{22.4L/mol}$=1.75mol��
����������CO�����ʵ���Ϊx��CO2�����ʵ���Ϊy��
����$\left\{\begin{array}{l}{x+y=1.75}\\{28x+44y=61}\end{array}\right.$��
���x=1mol��y=0.75mol��
COռ����������������Ϊ���ʵ���������Ϊ$\frac{1mol}{1.75mol}$��100%��57.1%��
�ʴ�Ϊ��1.75��57.1��
��4���ڱ�״���µ�448mlij��������ʵ���Ϊ$\frac{0.448L}{22.4L/mol}$=0.02mol����Ħ������M=$\frac{1.28g}{0.02mol}$=64g/mol��
��g/molΪ��λʱ����Է���������ֵ�ϵ�����Ħ�����������Ը��������Է�������Ϊ64���ʴ�Ϊ��64��
��5������Na��Mg��Al��Ԫ�ص������ֱ�Ϊ23g��16g��9g��
n��NaCl��=n��Na��=$\frac{23g}{23g/mol}$=1mol��
n��MgCl2��=n��Mg��=$\frac{16g}{24g/mol}$=$\frac{2}{3}$mol��
n��AlCl3��=n��Al��=$\frac{9g}{27g/mol}$=$\frac{1}{3}$mol��
��n��NaCl����n��MgCl2����n��AlCl3��=1mol��$\frac{2}{3}$mol��$\frac{1}{3}$mol=3��2��1��
��AlCl3�����ʵ���Ϊx����
1mol=3x+2x��2+3x
���x=0.1
�ʻ�����������Ϊ0.1mol��3��58.5g��mol+0.1mol��2��95g/mol+0.1mol��133.5g/mol=49.9g��
�ʴ�Ϊ��3��2��1��49.9��
���� ���⿼�����ʵ����йؼ��㣬��Ŀ�����̲ģ�������ǿ�������ڻ���֪ʶ�Ĺ��̣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
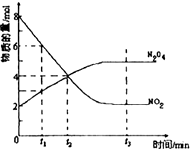 һ���¶��£����ݻ�Ϊ1L���ܱ������з���2molN2O4��8molNO2���������·�Ӧ2NO2������ɫ���TN2O4����ɫ����H��0��Ӧ��NO2��N2O4�����ʵ����淴Ӧʱ��仯��������ͼ��������Ҫ������
һ���¶��£����ݻ�Ϊ1L���ܱ������з���2molN2O4��8molNO2���������·�Ӧ2NO2������ɫ���TN2O4����ɫ����H��0��Ӧ��NO2��N2O4�����ʵ����淴Ӧʱ��仯��������ͼ��������Ҫ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Fe2+��Na+��NO3-��Cl- | B�� | Mg2+��NH4+��SO42-��NO3- | ||
| C�� | H+��K+��Cl-��CH3COO- | D�� | K+��Mg2+��CO32-��OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ӻ��������Ŀ����״�Ͳ����ӻ���ԭ�ӹ����Ŀ����״����ͬ | |
| B�� | �����ӻ��������״��������ȣ� NH3��N�ǵ����ӻ� | |
| C�� | ���ӻ�����ڿռ�Ӧ������ӶԻ������ۣ���ʹ�ų�����С | |
| D�� | sp3�ӻ����Ӧ��ͬԭ����������ͬ��s��p����ӻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��֪2H2��g��+O2��g���T2H2O��g����H=-483.6 kJ/mol����������ȼ����Ϊ241.8 kJ/mol | |
| B�� | ��֪NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l����H=-57.3 kJ/mol����0.5 mol NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�������С��28.65kJ | |
| C�� | ��ȼ���ϵ��Ϊ1��ȼ�շ�Ӧ���ʱ伴Ϊ�ÿ�ȼ���ȼ���� | |
| D�� | ��֪2C��s��+2O2��g���T2CO2��g����H=a kJ/mol��2C��s��+O2��g���T2CO��g����H=b kJ/mol����a��b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ca��ClO��2��Һ��ͨ������Ķ����������壺ClO-+SO2+H2O=HClO+HSO3- | |
| B�� | �ø��������Һ�ζ����2MnO4-+16H++5C2O42-=2Mn2++10CO2��+8H2O | |
| C�� | ϡ�����м���������ۣ�Fe+4H++NO3-=Fe3++NO��+2H2O | |
| D�� | ��̼�������Һ�м������������������Һ��Ca2++2OH-+2HCO3-=CaCO3��+2H2O+CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2.0gH218O��D2O�Ļ����������������ΪNA | |
| B�� | ���³�ѹ�£�4.4g��ȩ�����Ҽ���ĿΪ0.7 NA | |
| C�� | ��״���£�5.6L CO2������Na2O2��Ӧת�Ƶĵ�����Ϊ0.5 NA | |
| D�� | 50ml 12mol/L����������MnO2���ȣ�ת�Ƶĵ�����Ϊ0.3 NA |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com