


 ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 +3NaOH��C17H35COONa+
+3NaOH��C17H35COONa+
 +3NaOH��C17H35COONa+
+3NaOH��C17H35COONa+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��Ȼ��֬A�ķ���ʽΪC57H106O6��1mol����֬ˮ��ɵõ�1mol���͡�1mol������֬����B��2molֱ������֬����C�����ⶨB����Է�������Ϊ280��ԭ�Ӹ�����ΪC��H��O��9��16��1��
��1��д��B�ķ���ʽ��________��
��2��д��C�Ľṹ��ʽ��________________��C��������__________��
��3��д����5��̼ԭ�ӵ�C��ͬϵ���ͬ���칹��Ľṹ��ʽ��__________��
��.RCH==CHR�������KMnO4��Һ���Ⱥ��ữ������˫�������������
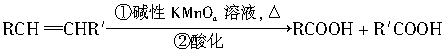 �������ø÷�Ӧ�IJ��ﷴ�ƺ�̼̼˫��������Ľṹ���ڴ��������£�1mol������֬����B��?1molH2��Ӧ�����õ�D��E�Ļ���D��E��Ϊͬ���칹�塣��D��E�Ļ���������KMnO4��Һ�����ữ�õ��������ֲ��
�������ø÷�Ӧ�IJ��ﷴ�ƺ�̼̼˫��������Ľṹ���ڴ��������£�1mol������֬����B��?1molH2��Ӧ�����õ�D��E�Ļ���D��E��Ϊͬ���칹�塣��D��E�Ļ���������KMnO4��Һ�����ữ�õ��������ֲ��
HCOOC��(CH2)10��COOH��CH3��(CH2)7��COOH
HCOOC��(CH2)7��COOH��CH3��(CH2)4��COOH
��4��д��D��E�Ľṹ��ʽ��__________________________________________________��
��5��д��B�Ľṹ��ʽ��_____________________________________________________��
��6��д����Ȼ��֬A��һ�ֿ��ܽṹ��ʽ��_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ����ʮ���и߶���ѧ����ĩ��ѧ�Ծ� ���ͣ������
(8��).ij��Ȼ��֬A�ķ���ʽΪC57H106O6��1 mol����֬ˮ��ɵõ�1 mol���͡�1 mol������֬����B��2molֱ������֬����C�����ⶨB����Է�������Ϊ280��ԭ�Ӹ�����ΪC��H��O=9��16��1��
(1)д��B�ķ���ʽ�� �� ��
(2)д��C�Ľṹ��ʽ�� �� ��C�����ƣ� �� ��
(3)д������5��̼ԭ�ӵ�Cͬϵ���ͬ���칹��Ľṹ��ʽ�� �� ��(�м���д����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ����ĩ��ѧ�Ծ� ���ͣ������
(8��).ij��Ȼ��֬A�ķ���ʽΪC57H106O6 ��1 mol����֬ˮ��ɵõ�1 mol���͡�1 mol������֬����B��2molֱ������֬����C�����ⶨB����Է�������Ϊ280��ԭ�Ӹ�����ΪC��H��O=9��16��1��
(1)д��B�ķ���ʽ�� �� ��
(2)д��C�Ľṹ��ʽ�� �� ��C�����ƣ� �� ��
(3)д������5��̼ԭ�ӵ�Cͬϵ���ͬ���칹��Ľṹ��ʽ�� �� ��(�м���д����)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com