
��100 mL FeI2��Һ����ͨ��Cl2������������I2��Fe3����IO3-������Fe3����I2�����ʵ�����n(Cl2)�ı仯��ͼ��ʾ����ش��������⣺

��1����ͼ��֪��I����Fe2����I2�������ӵĻ�ԭ����ǿ������˳��Ϊ________��________��________��
��2����n(Cl2)��0.12 molʱ����Һ�е�������ҪΪ________________________________��
�ӿ�ʼͨ��Cl2��n(Cl2)��0.12 molʱ���ܷ�Ӧ�Ļ�ѧ����ʽΪ______________________��
��3������Һ��n(Cl��)��n(IO3-)��8��1ʱ��ͨ���Cl2�ڱ�״���µ����Ϊ________��
��1��I����Fe2����I2
��2��Fe2����Fe3����Cl����5FeI2��6Cl2=5I2��2FeCl3��3FeCl2
��3��8.96 L
����������1������ͼ���֪������I����������Ȼ����Fe2�������Ի�ԭ��˳��ΪI����Fe2����I2����2����ͼ���֪n(I2)��0.1 mol������n(FeI2)��0.1 mol��n(I��)��0.2 mol����ͨ��0.12 mol Cl2��0.24 mol Clʱ��I��ȫ����������Fe2����0.04 mol��������������Һ�е�������Ҫ�У�Fe2����Fe3����Cl���������ʵ����ֱ�Ϊ0.06 mol��0.04 mol��0.24 mol��
I2Ϊ0.1 mol��
n(I2)��n(FeCl3)��n(FeCl2)��0.1��0.04��0.06��5��2��3
����ʽΪ5FeI2��6Cl2=5I2��2FeCl3��3FeCl2��
��3��Fe2�� �� 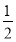 Cl2����I��������
Cl2����I��������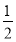 Cl2��I2��2IO3-��5Cl2
Cl2��I2��2IO3-��5Cl2
0.1 mol 0.05 mol 0.2 mol 0.1 mol x 2x 5x
������ã�
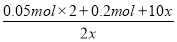 ��8
��8
x��0.05 mol
V(Cl2)��(0.05 mol��0.1 mol��5��0.05 mol)��22.4 L��mol��1��8.96 L��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ���ָ�ϰ��ʱ��ѵ ר��14�ۺ�ʵ����̽����ϰ��B�������棩 ���ͣ�ʵ����
Ϊ��̽��SO2��Na2O2�ķ�Ӧ�Ƿ�������CO2��Na2O2�ķ�Ӧ����ͬѧ�������ͼ��ʾ��ʵ��װ�ã��ش��������⣺

��1���ƿ������������ǵ�ľ������C�Թܿڣ�δ��ľ����ȼ����ͬѧ�����ΪSO2��Na2O2�ķ�Ӧ��ͬ��CO2���밴��ͬѧ�Ĺ۵�д����Ӧ�Ļ�ѧ����ʽ ��
��2����ͬѧ��Ϊ���۷�Ӧԭ����Σ����ն���O2��������ͬѧ�������� ��������ͬѧ�Ĺ۵㣬��װ�������ĸĽ���
��
��3������Na2O2��ȫ��Ӧ����Ӧ��Bװ���й�������������ǣ���Na2SO3����Na2SO4����Na2SO3��Na2SO4��
�����ʵ�鷽�����飬д��ʵ�鲽���Լ�Ԥ������ͽ��ۣ�����±���
��ѡ�Լ���2 mol��L��1 HCl��Һ��1 mol��L��1 HNO3��Һ��1 mol��L��1 BaCl��Һ��1 mol��L��1 Ba��NO3��2��Һ��0.01 mol��L��1 KMnO4������Һ��
ʵ�鲽�� | Ԥ������ͽ��� |
����1��ȡB�е�����������Ʒ���Թ��У��μ���������ˮ���ܽ⣬Ȼ��ȡ��������Һ�ֱ������������Թ��� | ������ȫ�ܽ� |
����2�������Թ��м��� ���ٵμ� | �� |
��֤���������к�Na2SO4 |
|
����3�������Թ��� |
|
| �� �� |
��֤������������Na2SO3���� |
|
|
|
��˵����������û��Na2SO3�� |
|
��4�����������������ƺ����IJⶨ��
��ȡa g���������Ƴ�100 mL��Һ��ȡ10.00 mL����Һ����ƿ�У����뼸�ε�����Һ��ָʾ������0.010 0 mol��L��1��ˮ���еζ����ζ��յ�����Ϊ ����¼���ݣ��ظ��ζ�2�Σ�ƽ�����ĵ�ˮ20.00 mL��
�����㣺���������������Ƶ���������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ���ָ�ϰ��ʱ��ѵ ר��12�л���Ľṹ��������ϰ���������棩 ���ͣ�ѡ����
����������ȷ���ǣ�������
A������ϩ�Ľṹ��ʽΪCH2CHCl
B�������ʵ�ˮ�����Ϊ�����ᣬ���۵�ˮ�����Ϊ�����ǣ������ڴ�����
C����ϩ��ˮ��һ�������·����ӳɷ�Ӧ��������Ǵ�����
D����5��̼ԭ�ӵ��л��ÿ�������������γ�4��̼̼����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ���ָ�ϰ��ʱ��ѵ ר��10�ǽ���Ԫ�ص��ʼ���������ϰ���������棩 ���ͣ�ʵ����
ij��ѧ��ѧʵ��С��Ϊ��֤���ͱȽ�SO2����ˮ��Ư���ԣ��������ͼ��ʾװ�ã�
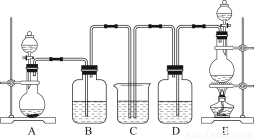
��1��ʵ���ҳ���װ��E�Ʊ�Cl2��ָ���÷�Ӧ��Ũ���������ֳ���������________________��
��2����Ӧ��ʼ����B��D��������ƿ�е�Ʒ����Һ����ɫ��ֹͣͨ��������ˮԡ��B��D��������ƿ���ȡ���������ƿ�е�����ֱ�Ϊ��B____________________��D____________________��
��3��װ��C��������______________________________________________________��
��4����ʵ��С��ļס�����λͬѧ����������������װ�ð�ͼ��ʾװ�ü�������ʵ�飺
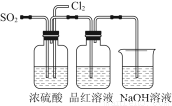
ͨ��һ��ʱ���ͬѧʵ�������Ʒ����Һ��������ɫ������ͬѧ��ʵ��������Ʒ����Һ��ʱ������Ʊ��Խ��Խdz��
�Ը��ݸ�ʵ��װ�ú�����ͬѧ��ʵ�����ش����⣺
��ָ�����������Ʒ����Һ֮ǰ���Ƚ�SO2��Cl2ͨ��Ũ�����е�Ŀ�ģ�________________________________________________________________________________________________________________________________________________��
���Է�����ͬѧʵ������У�Ʒ����Һ����ɫ��ԭ����____________________________________________________________________________________________________________________________________________________________________________________________________________________����������ӷ���ʽ˵����
������Ϊ��ͬѧ������������Ʒ����Һ���Խ��Խdz�ģ�
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ���ָ�ϰ��ʱ��ѵ ר��10�ǽ���Ԫ�ص��ʼ���������ϰ���������棩 ���ͣ�ѡ����
����ʵ����ʵ���ó�����Ӧ���ۺ������ǣ�������
ʵ����ʵ����
A��Cl2��ˮ��Һ���Ե���Cl2�ǵ����
B����ȼ�ŵ�þ������ʢ��CO2�ļ���ƿ�м���ȼ����ԭ�ԣ�Mg>C
C��SO2����ʹ����KMnO4��Һ��ɫSO2����Ư����
D�������۷���ϡHNO3�г�ַ�Ӧ����KSCN��Һ����������ϡHNO3���ܽ�Fe������Fe3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר����������ԭ��Ӧ��ϰ���������棩 ���ͣ�ѡ����
��һ������Fe��Fe2O3�Ļ�������250 mL��1.8 mol��L��1��HNO3��Һ�У�������������ȫ�ܽ���ڱ�״��������1.12 L NO(HNO3�Ļ�ԭ�������һ��)������Ӧ�����Һ�м���1.0 mol��L��1 NaOH��Һ����Ҫʹ��Ԫ����ȫ�������������NaOH��Һ���������ӦΪ(����)
A��300 mL B��400 mL C��450 mL D��500 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר��ʮ�������Һ��ϰ���������棩 ���ͣ������
ˮ��������ԴȪ����ҵ��ѪҺ�����е�������Ҫ�����ú�������ˮ����Ҫ������ˮԴ����Ⱦ��ͨ������ˮ��ֱ�Ӷ������壬Ҳ��ͨ��ʳ�������ũ����Σ��������
��ش��������⣺
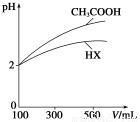
(1)��ˮ��100 ��ʱ��pH��6�����¶���1 mol��L��1��NaOH��Һ�У���ˮ�������
c(OH��)��________ mol��L��1��
(2)25 ��ʱ����ˮ�ĵ���ƽ����ϵ�м�������̼���ƹ��壬�õ�pHΪ11����Һ����ˮ�ⷽ��ʽΪ__________����ˮ�������c(OH��)��__________ mol��L��1��
(3)�����Ϊ100 mL��pH��Ϊ2��CH3COOH��һԪ��HX����ˮϡ������pH����Һ����Ĺ�ϵ����ͼ��ʾ����HX�ĵ���ƽ�ⳣ��________(��������������С��������������)CH3COOH�ĵ���ƽ�ⳣ����������__________________________________��
(4)����ƽ�ⳣ���Ǻ���������ʵ���̶�ǿ��������������֪��
��ѧʽ | ���볣��(25 ��) |
HCN | K��4.9��10��10 |
CH3COOH | K��1.8��10��5 |
H2CO3 | K1��4.3��10��7��K2��5.6��10��11 |
��25 ��ʱ���е�Ũ�ȵ�NaCN��Һ��Na2CO3��Һ��CH3COONa��Һ������Һ��pH�ɴ�С��˳��Ϊ____________________________��
����NaCN��Һ��ͨ��������CO2��������Ӧ�Ļ�ѧ����ʽΪ__________________
(5)25 ��ʱ����CH3COOH��CH3COONa�Ļ����Һ�У������pH��6������Һ��c(CH3COO��)��c(Na��)��________ mol��L��1(�ȷֵ)��c(CH3COO��)/c(CH3COOH)��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר��ʮ�ij����л��P��Ӧ����ϰ���������棩 ���ͣ�ѡ����
Һ��������Ҫ�ɷ��DZ��飬�����йر������������ȷ����(����)
A����ֱ��������������3��̼ԭ�Ӳ���һ��ֱ����
B���ڹ����������ܹ�����������ȡ����Ӧ
C������ȶ�����Һ��
D��1 mol������ȫȼ������5 mol O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר��ʮ���л���ѧ������ϰ���������棩 ���ͣ������
��������(clopidogrel,1)��һ����������ѪС��ۼ���ҩ�����ԭ�ϵIJ�ͬ����ҩ��ĺϳ�·��ͨ����������������2?�ȱ���ȩΪԭ�ϵĺϳ�·�����£�
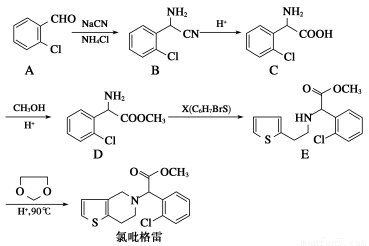
(1)����D�еĹ���������Ϊ________________��X�Ľṹ��ʽΪ____________��
(2)����C����һ�������·�Ӧ����һ�ֲ���ò�������к���3����Ԫ����д���÷�Ӧ�Ļ�ѧ����ʽ______________________________________
(3)D��E�ķ�Ӧ������________��Ӧ��
(4)д��A���ڷ����廯���������ͬ���칹��Ľṹ��ʽ��__________��
(5)��֪��CO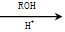 COROH
COROH COROR
COROR
�����Ҵ����״�Ϊ�л�ԭ���Ʊ������� ����Ҫ�����ķ�Ӧ������__________(��д���)�����ӳɷ�Ӧ������ȥ��Ӧ����ȡ����Ӧ����������Ӧ������ԭ��Ӧ��д���Ʊ�������
����Ҫ�����ķ�Ӧ������__________(��д���)�����ӳɷ�Ӧ������ȥ��Ӧ����ȡ����Ӧ����������Ӧ������ԭ��Ӧ��д���Ʊ������� �����һ����Ӧ_______________________________________________��
�����һ����Ӧ_______________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com