



 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1�U1 | B��2�U1 | C��1�U4 | D��1�U2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaOH | B��H2SO4 | C��NH3��H2O | D��KOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

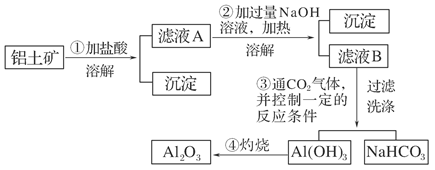
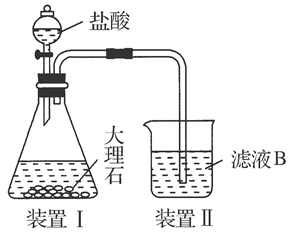
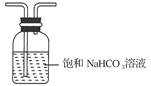
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
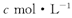 ������������ҺV mL,ʹ�������Ӹպó�����ȫ,�õ��ij�������Ϊn g���ٽ����ó����������������ٸı�Ϊֹ���õ�����p g0�����й�ϵ����ȷ����
������������ҺV mL,ʹ�������Ӹպó�����ȫ,�õ��ij�������Ϊn g���ٽ����ó����������������ٸı�Ϊֹ���õ�����p g0�����й�ϵ����ȷ����A��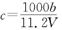 | B��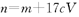 | C�� | D�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 + H2O ��C+ NaOH��B+NaCl ��E+ H2O��NaOH+F
+ H2O ��C+ NaOH��B+NaCl ��E+ H2O��NaOH+F ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
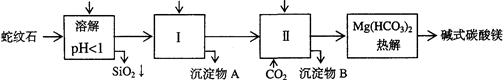
| �������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| ��ʼ����pH | 1.5 | 3.3 | 9.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��A��Һ�п��ܺ���H+��Mg2+��Al3+ Cl- |
| B��A��Һ����0.1molAl3+ |
| C��A��Һ��һ������AlO2- Na+���� |
| D��A��Һ����NaOH��Һ���ٷ�����������ѧ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na2SO4 | B��Na2SO4��MgSO4��Al2(SO4)3 | C��Na2SO4��MgSO4 | D��Na2SO4��NaAlO2 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com