
��ͼ��ʾ����������1��20��Ԫ���в���Ԫ����ɵĵ��ʻ��仯���ͼ�в��ַ�Ӧ����δ�г�����֪C��H����ɫ�д̼�����ζ�����壬D��һ�ֻ���ɫ�����嵥�ʣ�����J��������ˮ��������Ӧ�ں͢��ǻ��������е���Ҫ��Ӧ����Ӧ����ʵ�����Ʊ�����C����Ҫ������

��ش��������⣺
������E�ĵ���ʽ��____________��
�Ʒ�Ӧ�ݵĻ�ѧ����ʽΪ___________________________________________��
�Ƿ�Ӧ�۵�����Ϊ___________________________________________��
������A������Ԫ����ɣ�1molA��ˮ��Ӧ������1molB��2molC��A�Ļ�ѧʽΪ________��
��
��![]()
��Ca(OH)2��2NH4Cl![]() CaCl2��2NH3����2H2O
CaCl2��2NH3����2H2O
���а��̲���
��CaCN2
���������Ե�������ǡ�D�ǻ���ɫ�����嵥�ʡ���DΪ�������ɴ�չ�����Ʋ�J�Ǵ������Σ������Ŀ��������Σ�Ҳ�����Ǹ��Σ������ɴ��Ʋ�IΪ�������KΪ�Ȼ��C��HΪ��ɫ�д̼�����ζ���壬��C��H�ܻ�������G����GҲ�ܺ��������ﷴӦ���ʿ��Դ��Ʋ⣬C�ǰ�����H���Ȼ��⣬G���Ȼ�李���Ӧ����ʵ�����ư����ķ�����IΪCa��OH��2�� F��ˮ��Ӧ�����������ƣ�����Ϊ�����ƣ���B���Ը����·ֽ�ΪCaO����һ������E���²���ΪCaCO3��E��CO2�����ˣ���A��������ͼ��δ֪�ﶼ���˽��ۣ��ұ˴˿��Ի���ӡ֤����Ϊ��ȷ����A���Ʋ�Ϊ����Ĺؼ�Ҳ�������ѵ㡣��ˮ��Ӧ���ɰ��������ʣ������������ж��Ӵ������ǵ������ˮ�⡣������ǵ����ƣ�����ˮ������ǰ������������ƶ���̼��ƣ��ɼ����л���̼Ԫ�ء���������ĵ���С���еõ�֤ʵ����A�к���+2�۵�Ca��-3�۵�N��+4�۵�C�����A��B��C֮��Ϊ1��1��2 ����֪AΪCaCN2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ����������1��20��Ԫ���в���Ԫ����ɵĵ��ʻ��仯���ͼ�в��ַ�Ӧ�����Ͳ���δ�г�����֪C��D��E��H�dz�����������DΪ���ʡ���Ӧ�ں͢��ǻ��������е���Ҫ��Ӧ����Ӧ����ʵ�����Ʊ�����C����Ҫ��������Ӧ����J��ʧЧ��Ӧԭ����
��ش��������⣺
������L�ĵ���ʽΪ____________��E�ĽṹʽΪ_______________��
��D����Ԫ��λ�����ڱ�__________________������D�Ĺ�ҵ����Ϊ_______________��
�Ƿ�Ӧ�ݵĻ�ѧ����ʽΪ___________________________________________��
��Ӧ�����ӷ���ʽΪ___________________________________________��
������A������Ԫ����ɣ�1molA��ˮ��Ӧ������1molB��2molC��A�Ļ�ѧʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣���ͼ��ʾ����������1~20��Ԫ���в���Ԫ����ɵĵ��ʻ��仯���ͼ�в��ַ�Ӧ����δ�г�����֪C��H����ɫ�д̼�����ζ�����壬D��һ�ֻ���ɫ�����嵥�ʣ�����J������Ӿ����������Ӧ�ں͢��ǻ�����������Ҫ��Ӧ����Ӧ����ʵ�����Ʊ�����C����Ҫ������
��ش��������⣺
��1������E�ĵ���ʽ��__________��I��������ѧ������Ϊ��____________________������D����Ԫ�������ڱ��е�λ����____________________��
��2��G��ˮ��Һ��__________�ԣ������ӷ���ʽ��ʾ��ԭ��___________________________��
��3��д��E�����I��Һ��Ӧԭ���ӷ���ʽ______________________________��
��4��д��ʵ�����Ʊ�����C��Ӧ�Ļ�ѧ����ʽ______________________________����������C��ѡ��__________��������������ƣ���
��5��д����Ӧ�ܵĻ�ѧ����ʽ__________________________________________________��
��6����Ӧ�۵�����Ϊ______________________________��
��7����֪��7.4g I��ϡ��Һ��200mL 1 mol/L��H��Һ��Ӧ�ų�11.56kJ��������
д���÷�Ӧ���Ȼ�ѧ����ʽ________________________________________��
��8������A������Ԫ����ɣ�1 molA��ˮ��Ӧ������1 mol B��2 mol C��A��ѧʽΪ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭���ϸ߶��С����������ѧ�����꼶ȫ��ģ�⣨���ۣ���ѧ���� ���ͣ������
��16�֣���ͼ��ʾ����������1��20��Ԫ���в���Ԫ����ɵĵ��ʻ��ͼ�в��ַ�Ӧ����δ�г�����֪D��L��MΪ���嵥�ʣ�C��E��HΪ���廯�����Ӧ�ڡ��ܡ����ǻ��������е���Ҫ��Ӧ����Ӧ����ʵ�����Ʊ�����C����Ҫ������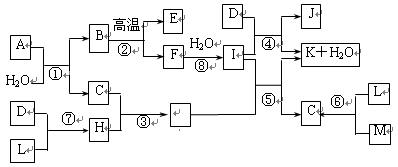
��ش��������⣺
��1������E�ĽṹʽΪ��__________��
��2�����ڻ��Ϸ�Ӧ����������������ԭ��Ӧ����__________���ñ����գ���
��3��C��H���۷е�Ƚϣ�C___H�����>������<����=������ԭ���ǣ�________________��
��4������A������Ԫ����ɣ�1molA��ˮ��Ӧ������1molB��2molC��A�Ļ�ѧʽΪ
______________��
��5����һ���¶��£��мס��� ���ݻ���ȵ��ܱ�������
���ݻ���ȵ��ܱ�������
I�����������ͨ��3 mol M��4 mol L����Ӧ�ﵽƽ��ʱ������C�����ʵ���Ϊa mol����ʱ��M��ת����Ϊ__________����������߷�Ӧ��L��ת���ʣ���ʵ�����������в�û�в��õĴ�ʩ��_____________��
�ٽ��ͺϳ����¶� ���ʵ�����ѹǿ
�۲��Ϸ��������C �ܼ�����ʴ���
II��������ͨ��2 mol C����ʹ��Ӧ�ﵽƽ��ʱ�������ʵ�Ũ����I�е�һ��ƽ��ʱ��ͬ������ʼʱ����ͨ��__________mol M��__________mol L��
III������ʼʱ��������ͨ��6mol M��8mol L���ﵽƽ��ʱ������C�����ʵ���Ϊb mol��
��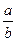 ________
________ 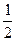 (ѡ�>������<����=������
(ѡ�>������<����=������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������и����������¿�����ѧ�Ծ� ���ͣ������
��14�֣���ͼ��ʾ����������1~20��Ԫ���в���Ԫ����ɵĵ��ʻ��仯���ͼ�в��ַ�Ӧ����δ�г�����֪C��H����ɫ�д̼�����ζ�����壬D��һ�ֻ���ɫ�����嵥�ʣ�����J������Ӿ����������Ӧ�ں͢��ǻ�����������Ҫ��Ӧ����Ӧ����ʵ�����Ʊ�����C����Ҫ������

��ش��������⣺
��1������E�ĵ���ʽ��__________��I��������ѧ������Ϊ��____________________������D����Ԫ�������ڱ��е�λ����____________________��
��2��G��ˮ��Һ��__________�ԣ������ӷ���ʽ��ʾ��ԭ��___________________________��[��Դ:ZXXK]
��3��д��E�����I��Һ��Ӧԭ���ӷ���ʽ______________________________��
��4��д��ʵ�����Ʊ�����C��Ӧ�Ļ�ѧ����ʽ______________________________����������C��ѡ��__________��������������ƣ���
��5��д����Ӧ�ܵĻ�ѧ����ʽ__________________________________________________��
��6����Ӧ�۵�����Ϊ______________________________��
��7����֪��7.4g I��ϡ��Һ��200mL 1 mol/L��H��Һ��Ӧ�ų�11.56kJ��������
д���÷�Ӧ���Ȼ�ѧ����ʽ________________________________________��
��8������A������Ԫ����ɣ�1 molA��ˮ��Ӧ������1 mol B��2 mol C��A��ѧʽΪ______________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com