
KI��Һ����������������������Ӧ�� ��������ʵ���¼��
ʵ���� | �� | �� | �� | �� | �� |
�¶�/�� | 30 | 40 | 50 | 60 | 70 |
��ɫʱ��/s | 160 | 80 | 40 | 20 | 10 |
�ش��������⣺
��1���÷�Ӧ�����ӷ���ʽΪ______________________��
��2����ʵ���Ŀ���� ___________________________��
��3��ʵ���Լ�����1 mol/L KI��Һ��0.1 mol/L H2SO4��Һ�⣬����Ҫ�������ɫ�Լ���__________________��ʵ������Ϊ��________________��
��4������ʵ������г�����Ҫ��3���������⣬��������Ʋ������________(ѡ����ĸ����
A���¶� B���Լ���Ũ�� C���Լ�������(�����
��5����Ҫ�������ԶԷ�Ӧ���ʵ�Ӱ���̽��ʵ�飬����ȡ�Ĵ�ʩ��___________________

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ��һ�ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵������ȷ����
A���Ȼ���ˮ��Һ�ڵ����������µ����Na����Cl��
B�����ᱵ������ˮ�������ᱵ���ڵ����
C��������̼����ˮ�ܵ��磬�ʶ�����̼���ڵ����
D������������������Һ����ԭ����ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ����������������и�һ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�õ�λ�������ܼ����������ʵ����ʵ�������ʾ����ҺŨ�Ƚ����������ʵ���Ũ�ȣ���λΪmol��kg��1����5 mol��kg��1��������Һ���ܶ�Ϊ1.3 g��mL��1�����������ʵ���Ũ�� Ϊ
Ϊ
A.3.85 mol��L��1 B��4.36 mol��L��1 C��5.25 mol��L��1 D��6.50mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ��һ�ϵڶ��ζο���ѧ�Ծ��������棩 ���ͣ�ѡ����
���ж��ڡ�Ħ������������ȷ����
A��Ħ���ǹ��ʿ�ѧ�罨����õ�һ��������
B��Ħ�������ʵ����ĵ�λ�����Ħ������Ϊmol
C��Ħ���������ʵĺ�������������ӵ�������ϵ����
D�������Ϲ涨��0.012kg̼ԭ�������е�̼ԭ����ĿΪ1mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ�߶��ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��(N2H4)�ǻ����������ȼ�ϣ�����N2O4��Ӧʱ��N2O4Ϊ�����������ɵ�����ˮ��������֪��N2(g)��2O2(g)===N2O4(g) ��H����8.7 kJ/mol��N2H4(g)��O2(g)===N2(g)��2H2O(g) ��H����534.0 kJ/mol�����б�ʾ�¸�N2O4��Ӧ���Ȼ�ѧ����ʽ����ȷ����
A��2N2H4(g)��N2O4(g)===3N2(g)��4H2O(g) ��H����542.7 kJ/mol
B��2N2H4(g)��N2O4(g)===3N2(g)��4H2O(g) ��H����1 059.3 kJ/mol
C��2N2H4(g)��N2O4(g)===3N2(g)��4H2O(g) ��H����1 076.7 kJ/mol
D��N2H4(g)��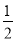 N2O4(g)===
N2O4(g)===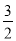 N2(g)��2H2O(g) ��H����1 076.7 kJ/mol
N2(g)��2H2O(g) ��H����1 076.7 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������������ѧ�߶���ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��Ũ��Ϊ0.1mol��L-1HF��Һ��ˮ����ϡ�ͣ����и���ʼ�ձ����������
A��c��H+�� B��Ka��HF�� C.c(F-��/c(H+�� D .c(F-��/c(HF��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������������ѧ�߶���ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��2A��B 3C��4D��Ӧ�У���ʾ�÷�Ӧ����������
3C��4D��Ӧ�У���ʾ�÷�Ӧ����������
A��v(A����0.5 mol/(L��s�� B��v(B���� 0.3 mol/(L��s��
C��v(C����0.8 mol/(L��s�� D��v(D����1 mol/(L��s��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ӱ�ʡ������ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ������
����һ��Ӧ�ù㷺�Ľ�������ҵ����Al2O3�ͱ���ʯ��Na3AlF6��������ڵ���Ƶá�
�����������Ҫ�ɷ���Al2O3��SiO2�ȡ���������������Al2O3���������£�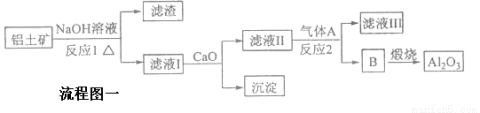
����өʯ��CaF2���ʹ���Ϊԭ���Ʊ�����ʯ���������£�

����ͼ��
�ش��������⣺
��1��д����Ӧ1�Ļ�ѧ����ʽ___________________��____________________��
��2��������A��������Ӧ2�����ӷ���ʽΪ ��
��3��д����D�Ʊ�����ʯ�Ļ�ѧ����ʽ ___________________________��
��4����ʯīΪ�������������������ʱ�����ĵ缫��Ӧʽ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�긣��ʡ�����и߶������У��ģ���ѧ�Ծ��������棩 ���ͣ�ѡ����
Ҫʹ�����б����������Դ�õ�������ã�������������з��ࡣ��ͼΪ���������־��������������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com