
| 3 | 2 |
| 3 |
| 2 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ǹ�ҵ����Ҫ��ԭ�ϡ�
��1���������ڹ�ҵ�ϳɰ� N2(g) + 3H2(g) ![]() 2NH3(g)����H = -92.2 kJ��mol-1��
2NH3(g)����H = -92.2 kJ��mol-1��
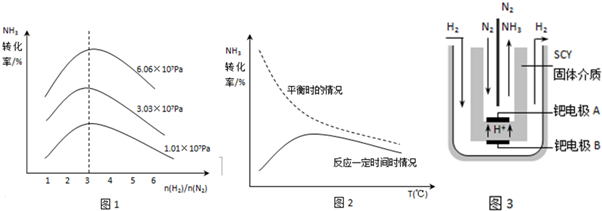
һ���¶��£����ݻ��㶨���ܱ������У�һ������N2��H2��Ӧ�ﵽƽ��ı�ijһ�����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ������t4 �pt5 �pt7ʱ������Ӧ��ʵ�������ı�ֱ���
t4 t5 t7 ��
���¶�ΪT��ʱ����2amolH2��amolN2����0.5L�ܱ������У���ַ�Ӧ����N2��ת����Ϊ50������ʱ�ų�����46.1 kJ������¶��·�Ӧ��ƽ�ⳣ��Ϊ ��
��2����ҵ�ϴ�����������Դ�ڽ�̿��ˮ���������µķ�Ӧ��
C(s) + H2O(g) ![]() H2(g) + CO(g) ��H = +131.3 kJ/mol
H2(g) + CO(g) ��H = +131.3 kJ/mol
�ٸ÷�Ӧ�ڵ����²����Է����е�ԭ���� ��
�ں��£����ݻ��ɱ���ܱ������У��������Ͽ��淴Ӧ��һ��ʱ��������������������仯ʱ���ܱ����÷�Ӧ�Ѵﵽƽ��״̬���У�����������ܶȣ��������������ѹǿ����������������ʵ����� ��CO���ʵ���Ũ�� �� ��
A��ֻ�Т� B��ֻ�Т�͢� C��ֻ�Т�͢�
D����͢� E��������
��3�����ݻ���ͬ�����ܱ�����A��B�У������¶�Ϊ423K��ͬʱ��A��B�зֱ����amol��bmol�⻯�⣨a��b������Ӧ��2HI(g)![]() H2(g)+I2(g) �ﵽƽ���ƽ��ʱI2��Ũ��c(I2)A c(I2)B ��ƽ��ʱHI�ķֽ��ʦ�A ��B ��ƽ��ʱH2�ڻ�������е��������A B ����д��������������������
H2(g)+I2(g) �ﵽƽ���ƽ��ʱI2��Ũ��c(I2)A c(I2)B ��ƽ��ʱHI�ķֽ��ʦ�A ��B ��ƽ��ʱH2�ڻ�������е��������A B ����д��������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��9�֣������ǹ�ҵ����Ҫ��ԭ�ϡ�
��1���������ڹ�ҵ�ϳɰ� N2(g) + 3H2(g) 2NH3(g)����H = -92.2 kJ��mol-1��
��һ���¶��£����ݻ��㶨���ܱ������У�һ������N2��H2��Ӧ�ﵽƽ��ı�ijһ�����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ������t2 �pt4 �pt5 �pt7ʱ������Ӧ��ʵ�������ı�ֱ���
t2 t4 t5 t7 ��
���¶�ΪT��ʱ����2amolH2��amolN2����0.5L�ܱ������У���ַ�Ӧ����N2��ת����Ϊ50������ʱ�ų�����46.1 kJ������¶��·�Ӧ��ƽ�ⳣ��Ϊ ��
��2�����ݻ���ͬ�����ܱ�����A��B�У������¶�Ϊ423K��ͬʱ��A��B�зֱ����1mol��2mol�⻯�����Ӧ��2HI(g)H2(g)+I2(g) �ﵽƽ���ƽ��ʱI2��Ũ��c(I2)A c(I2)B ��ƽ��ʱHI�ķֽ��ʦ�A ��B ��ƽ��ʱH2�ڻ�������е��������A B ����д��������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�콭��ʡ�γ���ѧ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
�����ǹ�ҵ����Ҫ��ԭ�ϡ�
��1���������ڹ�ҵ�ϳɰ� N2(g) + 3H2(g) 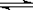 2NH3(g)����H =" -92.2" kJ��mol-1��
2NH3(g)����H =" -92.2" kJ��mol-1��
�� һ���¶��£����ݻ��㶨���ܱ������У�һ������N2��H2��Ӧ�ﵽƽ��ı�ijһ�����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ������t4 �pt5�pt7ʱ������Ӧ��ʵ�������ı�ֱ���
t4 t5 t7 ��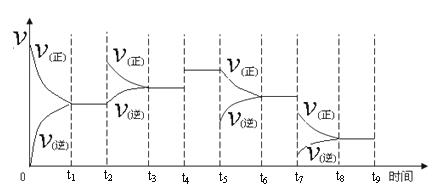
���¶�ΪT��ʱ����2amolH2��amolN2����0.5L�ܱ������У���ַ�Ӧ����N2��ת����Ϊ50������ʱ�ų�����46.1 kJ������¶��·�Ӧ��ƽ�ⳣ��Ϊ ��
��2����ҵ�ϴ�����������Դ�ڽ�̿��ˮ���������µķ�Ӧ��
C(s) + H2O(g) 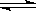 H2(g) + CO(g) ��H =" +131.3" kJ/mol
H2(g) + CO(g) ��H =" +131.3" kJ/mol
�ٸ÷�Ӧ�ڵ����²����Է����е�ԭ���� ��
�ں��£����ݻ��ɱ���ܱ������У��������Ͽ��淴Ӧ��һ��ʱ��������������������仯ʱ���ܱ����÷�Ӧ�Ѵﵽƽ��״̬���У�����������ܶȣ��������������ѹǿ����������������ʵ����� ��CO���ʵ���Ũ�� �� ��
A��ֻ�Т� B��ֻ�Т�͢� C��ֻ�Т�͢�
D����͢� E��������
��3�����ݻ���ͬ�����ܱ�����A��B�У������¶�Ϊ423K��ͬʱ��A��B�зֱ����amol��bmol�⻯�⣨a��b������Ӧ��2HI(g)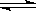 H2(g)+I2(g) �ﵽƽ���ƽ��ʱI2��Ũ��c(I2)A c(I2)B ��ƽ��ʱHI�ķֽ��ʦ�A ��B ��ƽ��ʱH2�ڻ�������е��������A B ����д��������������������
H2(g)+I2(g) �ﵽƽ���ƽ��ʱI2��Ũ��c(I2)A c(I2)B ��ƽ��ʱHI�ķֽ��ʦ�A ��B ��ƽ��ʱH2�ڻ�������е��������A B ����д��������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012�꽭���������������ѧ�߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��9�֣������ǹ�ҵ����Ҫ��ԭ�ϡ�
��1���������ڹ�ҵ�ϳɰ� N2(g) + 3H2(g)  2NH3(g)����H =" -92.2" kJ��mol-1��
2NH3(g)����H =" -92.2" kJ��mol-1��
��һ���¶��£����ݻ��㶨���ܱ������У�һ������N2��H2��Ӧ�ﵽƽ��ı�ijһ�����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ������t2 �pt4 �pt5�pt7ʱ������Ӧ��ʵ�������ı�ֱ���
t2 t4 t5 t7 ��
���¶�ΪT��ʱ����2amolH2��amolN2����0.5L�ܱ������У���ַ�Ӧ����N2��ת����Ϊ50������ʱ�ų�����46.1 kJ������¶��·�Ӧ��ƽ�ⳣ��Ϊ ��
��2�����ݻ���ͬ�����ܱ�����A��B�У������¶�Ϊ423K��ͬʱ��A��B�зֱ����1mol��2mol�⻯�����Ӧ��2HI(g) H2(g)+I2(g) �ﵽƽ���ƽ��ʱI2��Ũ��c(I2)A c(I2)B ��ƽ��ʱHI�ķֽ��ʦ�A ��B ��ƽ��ʱH2�ڻ�������е��������A B ����д��������������������
H2(g)+I2(g) �ﵽƽ���ƽ��ʱI2��Ũ��c(I2)A c(I2)B ��ƽ��ʱHI�ķֽ��ʦ�A ��B ��ƽ��ʱH2�ڻ�������е��������A B ����д��������������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com