
��10�֣�ʵ������Ҫ0.10 mol/L NaOH��Һ475mL��0.40 mol/L��������Һ500 mL��������������Һ����������ش��������⣺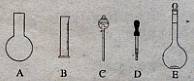
��1����ͼ��ʾ��������������Һ�϶�����Ҫ���� ������ţ�������������Һ�����õ��IJ��������� �����������ƣ���
��2�����dz������ƹ��̼���Ϊ���¸����裺
| A����ȴ | B������ | C��ϴ�� | D������ E���ܽ� F��ҡ�ȡ� G����Һ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��������2012������ڶ����¿���ѧ���� ���ͣ�058
ʵ������Ҫ0.10 mol/L��NaOH��Һ475 mL��0.40 mol/L��������Һ500 mL��������������Һ����������ش��������⣺
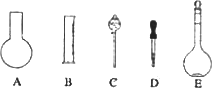
(1)��ͼ��ʾ��������������Һ�϶�����Ҫ����________(�����)������������Һ�����õ��IJ���������________(����������)��
(2)���dz������ƹ��̼���Ϊ���¸����裺
A����ȴ
B������
C��ϴ��
D������
E���ܽ�
F��ҡ�ȣ�
G����Һ
����ȷ�IJ���˳��Ӧ��________(����������)��
(3)�����ʵ����������ƽ��ȡNaOH������Ϊ________g����ʵ����������������ȷ��������ʱ�����ӿ̶��ߣ�����������ҺŨ��________0.1 mol/L(����¡��������ڡ���С�ڡ�����ͬ)������Һʱ������ƿ��������������ˮ����������ҺŨ��________0.1 mol/L��
(4)���ݼ����֪��������������A��98�����ܶ�Ϊ1184 g/cm3��Ũ��������Ϊ________mL(����һλС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ������ѧ�ڵ������¿���ѧ�Ծ� ���ͣ�ʵ����
��10�֣�ʵ������Ҫ0.10 mol/L NaOH��Һ475mL��0.40 mol/L��������Һ500 mL��������������Һ����������ش��������⣺
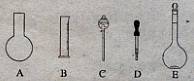
��1����ͼ��ʾ��������������Һ�϶�����Ҫ���� ������ţ�������������Һ�����õ��IJ��������� �����������ƣ���
��2�����dz������ƹ��̼���Ϊ���¸����裺
A����ȴ B������ C��ϴ�� D������ E���ܽ� F��ҡ�ȡ� G����Һ
��ȷ�IJ���˳��Ӧ�� �����������ţ���
��3�������ʵ����������ƽ��ȡNaOH������Ϊ g����ʵ����������������ȷ��������ʱ�����ӿ̶��ߣ�����������ҺŨ�� 0.lmol/L������¡��������ڡ���С�ڡ�����ͬ��������Һʱ������ƿ��������������ˮ����������ҺŨ�� 0.lmol��L��
��4�����ݼ����֪��������������A 98%���ܶ�Ϊ1184g/cm3��Ũ��������Ϊ____mL������һλС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�ʵ������Ҫ0.10 mol/L NaOH��Һ475mL��0.40mol/L��������Һ500 mL��������������Һ����������ش��������⣺
��1����ͼ��ʾ��������������Һ�϶�����Ҫ���� ������ţ�������������Һ�����õ��IJ��������� �����������ƣ���
��2�����dz������ƹ��̼���Ϊ���¸����裺
A����ȴ B������ C��ϴ�� D������ E���ܽ� F��ҡ�ȡ� G����Һ
��ȷ�IJ���˳��Ӧ�� �����������ţ���
��3�������ʵ����������ƽ��ȡNaOH������Ϊ g����ʵ����������������ȷ��������ʱ�����ӿ̶��ߣ�����������ҺŨ�� 0.lmol/L������¡��������ڡ���С�ڡ�����ͬ��������Һʱ������ƿ��������������ˮ����������ҺŨ�� 0.lmol��L��
��4�����ݼ����֪��������������A 98%���ܶ�Ϊ1184g/cm3��Ũ��������Ϊ____mL������һλС������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com