
��ʽ̼��ͭ�ɱ�ʾΪ��xCuCO3��yCu(OH) 2��zH2O,�ⶨ��ʽ̼��ͭ��ɵķ����ж��֡�
![]() ��1���ֲ���������ԭ������ش��������⣺
��1���ֲ���������ԭ������ش��������⣺![]()
![]()
![]()
![]()
![]() ��д��xCuCO3��yCu(OH) 2��zH2O��������Ӧ�Ļ�ѧ����ʽ ��
��д��xCuCO3��yCu(OH) 2��zH2O��������Ӧ�Ļ�ѧ����ʽ ��
![]() ������װ�������������������Ӷ��ɣ������������������˳���ǣ����������ӿ���ĸ��ţ���
������װ�������������������Ӷ��ɣ������������������˳���ǣ����������ӿ���ĸ��ţ���
![]() ��a������ ���� ������ ���� ������ ���� ������ ���� ������ ���� ������l��
��a������ ���� ������ ���� ������ ���� ������ ���� ������ ���� ������l��
 |
![]()
![]()
![]()
![]()
![]() �۳�ȡ23.9gij��ʽ̼��ͭ��Ʒ����ַ�Ӧ��õ�12.7g���������4.4g������̼��7.2gˮ������Ʒ�Ľᾧˮ����Ϊ g����ѧʽΪ ��
�۳�ȡ23.9gij��ʽ̼��ͭ��Ʒ����ַ�Ӧ��õ�12.7g���������4.4g������̼��7.2gˮ������Ʒ�Ľᾧˮ����Ϊ g����ѧʽΪ ��
![]() ��2��ijͬѧ�Ե���������������������ȫ�����������ⶨ��ʽ̼��ͭ����ɣ�����Ϊ�Ƿ���У���˵�����ɡ� ��
��2��ijͬѧ�Ե���������������������ȫ�����������ⶨ��ʽ̼��ͭ����ɣ�����Ϊ�Ƿ���У���˵�����ɡ� ��
(1)��xCuCO3��yCu(OH)2��zH2O+(x+y)H 2 = (x+y)Cu+ xCO2+(x+2y+z)H2O
![]() ��a��k,j��gf(hi)��de(ed)��hi(gf)��bc(cb)��l
��a��k,j��gf(hi)��de(ed)��hi(gf)��bc(cb)��l
![]() ��1.8 CuCO3��Cu(OH) 2��H2O
��1.8 CuCO3��Cu(OH) 2��H2O
![]() (2)���� ���ݷ�ӦxCuCO3��yCu(OH) 2��zH2O=(x+y)CuO+ xCO2��+(y+z)H2O��,���ݼ�ʽ̼��ͭ��CuO��CO2��H2O�����������������������������ɼ��������ɡ�
(2)���� ���ݷ�ӦxCuCO3��yCu(OH) 2��zH2O=(x+y)CuO+ xCO2��+(y+z)H2O��,���ݼ�ʽ̼��ͭ��CuO��CO2��H2O�����������������������������ɼ��������ɡ�
��1������ļ�ʽ̼��ͭ��������Ӧ����һ�����ѵ���Ϣ����ʵϸ��һ��ֻҪ��������ΪCuCO3��Cu(OH)2���ȷֽ�����CuO����������Ӧ���ڢ��������Խ�������ڢ�Ҫ�ܷ������ⶨ��Ӧ��CO2��H2O��������˶���������������������ѡ���dz��Ȼ����ˮ��������ֹ�Ժ����ⶨӰ��Ϳ����ˣ���Ϊ�ⶨH2O��CO2�ֱ���Ũ����ͼ�ʯ���ǹ̶��ġ�
![]() ��2����ʵ�ڷ�����1���ٷ���ʽ��дʱ��õ��˼�ʽ̼��ͭ�ȷֽⷽ��ʽ�� xCuCO3��yCu(OH) 2��zH2O=(x+y)CuO+ xCO2��+(y+z)H2O�����Լӷ�����֪�����ݼ�ʽ̼��ͭ��CuO��CO2��H2O�����������������������������ɼ��������ɡ�
��2����ʵ�ڷ�����1���ٷ���ʽ��дʱ��õ��˼�ʽ̼��ͭ�ȷֽⷽ��ʽ�� xCuCO3��yCu(OH) 2��zH2O=(x+y)CuO+ xCO2��+(y+z)H2O�����Լӷ�����֪�����ݼ�ʽ̼��ͭ��CuO��CO2��H2O�����������������������������ɼ��������ɡ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣���ʽ̼��ͭ�ɱ�ʾΪ��xCuCO3?yCu(OH) 2?zH2O,�ⶨ��ʽ̼��ͭ��ɵķ����ж��֡�
![]() ��1���ֲ���������ԭ������ش��������⣺
��1���ֲ���������ԭ������ش��������⣺
![]() ��д��xCuCO3?yCu(OH) 2?zH2O��������Ӧ�Ļ�ѧ����ʽ���������������������� ��
��д��xCuCO3?yCu(OH) 2?zH2O��������Ӧ�Ļ�ѧ����ʽ���������������������� ��
![]() ������װ�������������������Ӷ��ɣ������������������˳���ǣ����������ӿ���ĸ��ţ���
������װ�������������������Ӷ��ɣ������������������˳���ǣ����������ӿ���ĸ��ţ���
![]() ��a������ ���� ������ ���� ������ ���� ������ ���� ������ ���� ������l��
��a������ ���� ������ ���� ������ ���� ������ ���� ������ ���� ������l��
![]()
![]()
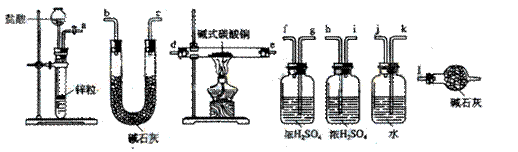
![]() �۳�ȡ23.9gij��ʽ̼��ͭ��Ʒ����ַ�Ӧ��õ�12.7g���������4.4g������̼��7.2gˮ������Ʒ�Ľᾧˮ����Ϊ�������� g����ѧʽΪ���������� ��
�۳�ȡ23.9gij��ʽ̼��ͭ��Ʒ����ַ�Ӧ��õ�12.7g���������4.4g������̼��7.2gˮ������Ʒ�Ľᾧˮ����Ϊ�������� g����ѧʽΪ���������� ��
![]() ��2��ijͬѧ�Ե���������������������ȫ�����������ⶨ��ʽ̼��ͭ����ɣ�����Ϊ�Ƿ���У���˵�����ɡ��������������������� ����
��2��ijͬѧ�Ե���������������������ȫ�����������ⶨ��ʽ̼��ͭ����ɣ�����Ϊ�Ƿ���У���˵�����ɡ��������������������� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣���ʽ̼��ͭ�ɱ�ʾΪ��xCuCO3��yCu��OH�� 2��zH2O,�ⶨ��ʽ̼��ͭ��ɵķ����ж��֡�
��1���ֲ���������ԭ������ش��������⣺
��д��xCuCO3��yCu��OH�� 2��zH2O��������Ӧ�Ļ�ѧ����ʽ ��
������װ�������������������Ӷ��ɣ������������������˳���ǣ����������ӿ���ĸ��ţ���
��a������ ���� ������ ���� ������ ���� ������ ���� ������ ���� ������l��
�۳�ȡ23��9gij��ʽ̼��ͭ��Ʒ����ַ�Ӧ��õ�12��7g���������4��4g������̼��7��2gˮ������Ʒ�Ľᾧˮ����Ϊ g����ѧʽΪ ��
��2��ijͬѧ�Ե���������������������ȫ�����������ⶨ��ʽ̼��ͭ����ɣ�����Ϊ�Ƿ���У���˵�����ɡ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ����ϼκ�һ�и���1�·ݸ߿�Ѻ�⻯ѧ�Ծ� ���ͣ�ʵ����
��8�֣���ʽ̼��ͭ�ɱ�ʾΪ��xCuCO3��yCu��OH�� 2��zH2O,�ⶨ��ʽ̼��ͭ��ɵķ����ж��֡�
 ��1���ֲ���������ԭ������ش��������⣺
��1���ֲ���������ԭ������ش��������⣺
��д��xCuCO3��yCu��OH�� 2��zH2O��������Ӧ�Ļ�ѧ����ʽ ��
 ������װ�������������������Ӷ��ɣ������������������˳���ǣ����������ӿ���ĸ��ţ���
������װ�������������������Ӷ��ɣ������������������˳���ǣ����������ӿ���ĸ��ţ���
��a������ ���� ������ ���� ������ ���� ������ ���� ������ ���� ������l��


�۳�ȡ23��9gij��ʽ̼��ͭ��Ʒ����ַ�Ӧ��õ�12��7g���������4��4g������̼��7��2gˮ������Ʒ�Ľᾧˮ����Ϊ g����ѧʽΪ ��
 ��2��ijͬѧ�Ե���������������������ȫ�����������ⶨ��ʽ̼��ͭ����ɣ�����Ϊ�Ƿ���У���˵�����ɡ�
��
��2��ijͬѧ�Ե���������������������ȫ�����������ⶨ��ʽ̼��ͭ����ɣ�����Ϊ�Ƿ���У���˵�����ɡ�
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com