
| |||||||||||||||||||||||||||||
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

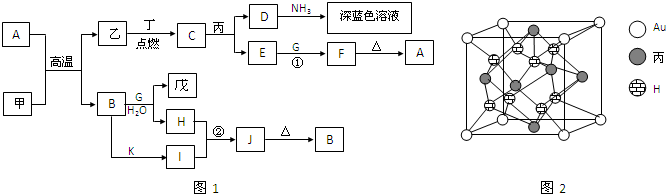
ͼ������������������еIJ���δ���������߲���ʾ��ѧ������Ӽ���������X��Y������ͬҲ���Բ�ͬ����֪��
�ټס�����ͬһ�ྦྷ���еĸ��Խṹ��Ԫ�����ʼ������ҷ����û���Ӧ
�ڱ����������������Ӻ��е����ĵ����������б�������ͬһ�ྦྷ���еķ��ӻ���Ӽ���
�۳����¶���Һ̬���ܲ������ֵȵ�����������
������������
(1)�����ҷ����û���Ӧ�Ļ�ѧ����ʽ��________________________________________��
(2)д��Һ̬���������ֵȵ������ӵĵ��뷽��ʽ��______________________________��
(3)����Ŀǰ��Ҫ����Դ�����Ͷ��ڴ������������µõ����ֿ�ȼ�Ե����壬�䷴Ӧ�Ļ�ѧ����ʽ��___________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ס��ҡ������춼������ͼ��ʾ�Ľṹ��ṹ��Ԫ��ͼ������������������еIJ���δ������ϸ���飩�߲���ʾ��ѧ������Ӽ���������X��Y������ͬҲ���Բ�ͬ����֪�ס�����ͬ�ྦྷ���еĸ��Խṹ��Ԫ�����ʼ������ҷ����û���Ӧ�������������������Ӻ��е����ĵ����������б��붡����������ͬ�������»����ﶡ��Һ̬�����������ӡ�
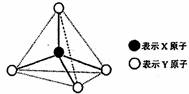
��1�����ĵ���ʽ�� ������̬ʱ�ľ�������Ϊ ��
��2�������������������Һ��pH ��
A��>7 B��=7 C��<7 D�����϶��п���
��3�������ҷ����û���Ӧ�Ļ�ѧ����ʽΪ ��
��4�������ӵĽṹʽ�� ������Ŀǰ��Ҫ����Դ���ʣ��ִ����ܵ���г��ñ���ȼ�ϵ�ص�ԭ�ϣ��ڼ��Խ��ʣ�KOH��Һ������£��为����Ӧ�ĵ缫����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ס��ҡ������춼������ͼ��ʾ�Ľṹ��ṹ��Ԫ��ͼ������������������еIJ���δ���������߲���ʾ��ѧ������Ӽ���������X��Y������ͬҲ���Բ�ͬ����֪��
�ס�����ͬһ�ྦྷ���еĸ��Խṹ��Ԫ�����ʼ������ҷ����û���Ӧ�������������������Ӻ��е����ĵ����������б�������ͬһ�ྦྷ���еķ��ӻ���Ӽ��ţ������¶���Һ̬���ܲ������ֵȵ����������ӣ����������ӡ�
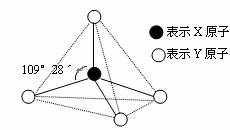
(1)д��Һ̬���������ֵȵ������ӵĵ��뷽��ʽ��
(2)�����º����������Һ����pH ��
A��>7 B����7 C��<7 D�����϶��п���
(3)����Ŀǰ��Ҫ����Դ
�ٱ��Ͷ��ڴ������������µõ���ȼ�Ե��������壬�䷴Ӧ�Ļ�ѧ����ʽ��
���ִ����ܵ���У����ñ���ȼ�ϵ�ص�ԭ�ϣ��ڼ��Խ���(KOH��Һ)������£���������Ӧ�ĵ缫����ʽΪ ����
(4)�ס��Ҹ��·�Ӧʱ���ױ��ƻ���1 mol���ۼ�����μӷ�Ӧ����Ϊ g��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com