

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���� | B��ͭ | C���� | D��Ǧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
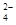 ��)���ֹ�(��C������Cl2��Ӧ�Ĺ�������)��ȡ���裬������µĹ������̣�
��)���ֹ�(��C������Cl2��Ӧ�Ĺ�������)��ȡ���裬������µĹ������̣�
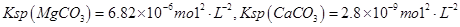 ������ô���ˮ��
������ô���ˮ��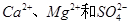 ��Ũ�Ⱦ�Ϊ0.01 mol��L-1������1 L�ô���ˮ������һ����Na2CO3��Һ�����ȳ��ֵij�����__________��
��Ũ�Ⱦ�Ϊ0.01 mol��L-1������1 L�ô���ˮ������һ����Na2CO3��Һ�����ȳ��ֵij�����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
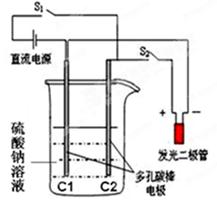
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
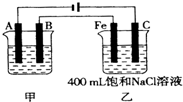
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��HCl��CuCl2��Ba(OH)2 | B��NaOH��CuSO4��H2SO4 |
| C��NaOH��H2SO4��Ba(OH)2 | D��NaBr��H2SO4��Ba(OH)2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������11��2 mL O2(��״��) | B������������32 mg Cu |
| C������������11��2 mL H2(��״��) | D�������������������ޱ仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
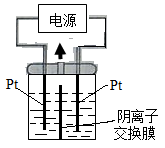
| A���Ҳ���ĵ缫����ʽ��2H2O+2e��==H2��+2OH�� |
| B��������ʱ���Ҳ���Һ�к���IO3�� |
| C�������ڷ�����Ӧ���ܻ�ѧ����ʽKI+3H2O======KIO3+3H2�� |
| D������������ӽ���Ĥ���������ӽ���Ĥ�������ڷ������ܻ�ѧ����ʽ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ô�ͭ����������ͭ������ |
| B�����Һ�ijɷֶ����ֲ��� |
C��������Ӧ��ֻ��Cu-2e- Cu2+ Cu2+ |
D��������Ӧ��ֻ��Cu2++2e- Cu Cu |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com