
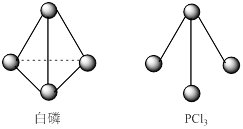


 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 H+ + CN���� K��1���� =" 4.9" ��10��10
H+ + CN���� K��1���� =" 4.9" ��10��10 NH4+ + OH���� K��2���� =" 1.8" ��10��5
NH4+ + OH���� K��2���� =" 1.8" ��10��5  H+ + OH���� Kw�� =" 1.0" ��10��14���NH3 + HCN
H+ + OH���� Kw�� =" 1.0" ��10��14���NH3 + HCN  NH4+ + CN���� ��Ӧƽ�ⳣ����Kֵ��_________________
NH4+ + CN���� ��Ӧƽ�ⳣ����Kֵ��_________________�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
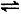 2SO3(g) ?��=��197kJ/mol���ڴ��¶��£���ס��������̶��ݻ����ܱ������зֱ�ͨ��2molSO2��1molO2��1mol SO2��0.5molO2����Ӧ�ﵽƽ��״̬ʱ�ų��������ֱ�ΪQ����Q���������й�ϵ��ȷ����
2SO3(g) ?��=��197kJ/mol���ڴ��¶��£���ס��������̶��ݻ����ܱ������зֱ�ͨ��2molSO2��1molO2��1mol SO2��0.5molO2����Ӧ�ﵽƽ��״̬ʱ�ų��������ֱ�ΪQ����Q���������й�ϵ��ȷ����| A��Q��=0.5Q�� | B��Q��< 0.5Q�� | C��Q��<Q��=197kJ | D��Q��=Q��<197kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2.44 kJ | B��4.88 kJ | C��44.0 kJ | D��88.0 kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��+1638KJ��mol�C1 | B���C1638KJ��mol�C1 | C��+126KJ��mol�C1 | D���C126KJ��mol�C1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH3OH��ȼ����Ϊ192.9 kJ��mol��1 |
| B��CH3OH��ȼ����Ϊ133��9 kJ��mol��1 |
| C��CH3OHת���H2�Ĺ���һ��Ҫ�������� |
| D�����ݢ���֪��Ӧ��CH3OH(l) ��1/2O2(g) �� CO2(g) ��2H2(g)��DH ����192.9 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
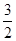 O2��g��=== Al2O3(s) ��H=" -1" 644.3 kJ? mol-1
O2��g��=== Al2O3(s) ��H=" -1" 644.3 kJ? mol-1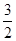 O2��g��=== Fe2O3(s) ��H=" -815.88" kJ? mol-1
O2��g��=== Fe2O3(s) ��H=" -815.88" kJ? mol-1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ�Т� | B��ֻ�Т� |
| C��ֻ�Тڢۢ� | D��ֻ�Т٢ڢ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com