
ijУ�о���ѧϰС�����������ͼ��ʾװ�ý��������й�ʵ�飮ʵ��ʱ����Һ©����A��μ�����ƿ��(ͼ������̨����������ȥ)��
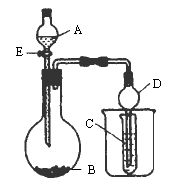
(1)��AΪŨ���ᣬBΪ��������Ԫ����ɵ�Ƭ״���ʣ����ڳ���������ˮ��Ӧ��CΪƷ����Һ��ʵ���й۲쵽��Һ��ɫ��ȥ����BΪ________(�ѧʽ)��Ȼ�����ձ��м����ˮ���ֿɹ۲쵽��������________��
(2)��BΪ��״����ʯ��CΪNa2CO3������Һ��ʵ���й۲쵽С�Թ�����Һ����ǣ�����A�����������е�________��
A��HCl
B��HNO3
C��H2SO4
(3)��BΪ��ʯ�ң�ʵ���й۲쵽C��Һ�����ɰ�ɫ�������ó�����������NaOH��Һ����������ϡ���ᣬ��A��________(�ѧ����)��C�����ɵij�����________(�ѧʽ)
 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д� �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���١��ۡ��ݡ��ڡ��� | B���ڡ��١��ۡ��ܡ��� | C���ڡ��ܡ��١��ۡ��� | D���ۡ��١��ڡ��ݡ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��1����AΪŨH2SO4��BΪ�������ڽ���Ԫ����ɵ�Ƭ״�������ʣ����ڳ���������ˮ��Ӧ��CΪƷ����Һ��ʵ���й۲쵽��Һ��ɫ��ȥ����BΪ__________��Ȼ�����ձ���ע���ˮ���ֿɹ۲쵽��������______________________��
��2����BΪ��״����ʯ��CΪC6H5ONa��Һ��ʵ���й۲쵽��Һ����ǣ���
����A���������е�________��
A.HCl B.HNO3 C.H2SO4 D.CH3COOH
��Ȼ�����ձ��м����ˮ���ɹ۲쵽��������_________________________��
��3����BΪ��ʯ�ң�ʵ���й۲쵽C��Һ�����ɳ�������������ܽ⣬����Һǡ�ó���ʱ���ر�F��Ȼ�����ձ��м���ˮ����ֹƬ�̣��۲쵽�Թܱ����й�������������A��___________��C��___________����ȩ�Ļ��Һ������D��ʵ���е�������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���Ĵ�ʡ��һ5���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
ijУ�о���ѧϰС�����������ʵ�鲽������ȡ�⣺
�ټ�H2O2�ڽ������ճɻң�����м�ˮ����ۼ�CC14�ܹ��ˢ��÷�Һ©����Һ�������IJ���˳��Ϊ
A���ڡ��ܡ��١��ۡ��� B���ڡ��١��ۡ��ܡ���
C���ۡ��١��ڡ��ݡ��� D���١��ۡ��ݡ��ڡ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10�궫ݸ�и�һ��ѧ����ĩ���Ի�ѧ�� ���ͣ�ѡ����
�����к��е�Ԫ�أ�ijУ�о���ѧϰС�����������ʵ�鲽������ȡ�⣺��ͨ�����������ڽ������ճɻң�����м�ˮ���裻�ۼ�CC14���ܹ��ˣ����÷�Һ©����Һ�������IJ���˳��Ϊ��
A���١��ۡ��ݡ��ڡ��� B���ڡ��١��ۡ��ܡ���
C���ڡ��ܡ��١��ۡ��� D���ۡ��١��ڡ��ݡ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com