
ºãΣ¨11000C£©ºãÈÝÃܱÕÈÝÆ÷Öз¢Éú·´Ó¦£ºNa2SO4(s)+4H2(g)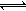 Na2S(s)+4H2O(g)
Na2S(s)+4H2O(g)
ÏÂÁÐ˵·¨Öв»ÕýÈ·µÄÊÇ
A.¸Ãƽºâ³£Êý±í´ïʽΪ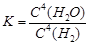
B.ÈÝÆ÷ÄÚÆøÌåµÄÖÊÁ¿»òѹǿ±£³Ö²»±ä£¬¾ù¿É˵Ã÷¸Ã·´Ó¦ÒѴﵽƽºâ״̬
C.ÈôNa2SO4×ãÁ¿£¬¸Ä±äÆðʼ³äÈëH2µÄŨ¶È£¬Ôò´ïµ½Æ½ºâʱµÄH2ת»¯Âʲ»±ä
D.Èô³õʼʱͶÈë2.84¿ËNa2SO4ÓëÒ»¶¨Á¿H2£¬·´Ó¦´ïµ½Æ½ºâʱÈÝÆ÷ÄÚ¹ÌÌå¹²ÓÐ2.264¿Ë£¬ÔòNa2SO4µÄת»¯ÂÊΪ45%
 ½×ÌݼÆËãϵÁдð°¸
½×ÌݼÆËãϵÁдð°¸
| Ä꼶 | ¸ßÖÐ¿Î³Ì | Ä꼶 | ³õÖÐ¿Î³Ì |
| ¸ßÒ» | ¸ßÒ»Ãâ·Ñ¿Î³ÌÍƼö£¡ | ³õÒ» | ³õÒ»Ãâ·Ñ¿Î³ÌÍƼö£¡ |
| ¸ß¶þ | ¸ß¶þÃâ·Ñ¿Î³ÌÍƼö£¡ | ³õ¶þ | ³õ¶þÃâ·Ñ¿Î³ÌÍƼö£¡ |
| ¸ßÈý | ¸ßÈýÃâ·Ñ¿Î³ÌÍƼö£¡ | ³õÈý | ³õÈýÃâ·Ñ¿Î³ÌÍƼö£¡ |
¿ÆÄ¿£º¸ßÖл¯Ñ§ À´Ô´£º2011-2012ѧÄêºÓÄÏÖÜ¿ÚÖصã¸ßÖÐËÄУ¸ß¶þÏÂѧÆÚµÚÒ»´ÎÁª¿¼»¯Ñ§¾í£¨´ø½âÎö£© ÌâÐÍ£ºµ¥Ñ¡Ìâ
ºãΣ¨11000C£©ºãÈÝÃܱÕÈÝÆ÷Öз¢Éú·´Ó¦£ºNa2SO4(s)+4H2(g) Na2S(s)+4H2O(g)
Na2S(s)+4H2O(g)
ÏÂÁÐ˵·¨Öв»ÕýÈ·µÄÊÇ
A.¸Ãƽºâ³£Êý±í´ïʽΪ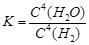
B.ÈÝÆ÷ÄÚÆøÌåµÄÖÊÁ¿»òѹǿ±£³Ö²»±ä£¬¾ù¿É˵Ã÷¸Ã·´Ó¦ÒѴﵽƽºâ״̬
C.ÈôNa2SO4×ãÁ¿£¬¸Ä±äÆðʼ³äÈëH2µÄŨ¶È£¬Ôò´ïµ½Æ½ºâʱµÄH2ת»¯Âʲ»±ä
D.Èô³õʼʱͶÈë2.84¿ËNa2SO4ÓëÒ»¶¨Á¿H2£¬·´Ó¦´ïµ½Æ½ºâʱÈÝÆ÷ÄÚ¹ÌÌå¹²ÓÐ2.264¿Ë£¬ÔòNa2SO4µÄת»¯ÂÊΪ45%
²é¿´´ð°¸ºÍ½âÎö>>
¹ú¼ÊѧУÓÅÑ¡ - Á·Ï°²áÁбí - ÊÔÌâÁбí
ºþ±±Ê¡»¥ÁªÍøÎ¥·¨ºÍ²»Á¼ÐÅÏ¢¾Ù±¨Æ½Ì¨ | ÍøÉÏÓк¦ÐÅÏ¢¾Ù±¨×¨Çø | µçÐÅթƾٱ¨×¨Çø | ÉæÀúÊ·ÐéÎÞÖ÷ÒåÓк¦ÐÅÏ¢¾Ù±¨×¨Çø | ÉæÆóÇÖȨ¾Ù±¨×¨Çø
Î¥·¨ºÍ²»Á¼ÐÅÏ¢¾Ù±¨µç»°£º027-86699610 ¾Ù±¨ÓÊÏ䣺58377363@163.com