
����Ŀ��Ϊ�о��Ҵ��ṹ���䲿�ֵĻ�ѧ���ʣ���������ʵ�顣����������⣺
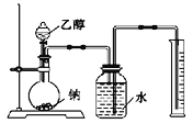
������ͼװ�����Ʋ��Ҵ��Ľṹʽ��
(1)ʵ��������Ҫ����ɺ�С�Ŀ�������ԭ���� _______________________________��
(2)����ʵ��ƽ�����Ҵ�1.15g���ռ���H2���ƽ��Ϊ0.28L(����ɱ�״̬)����ʵ�����ݿ��Ʋ�H2�����Ҵ�������________(������)����ԭ�ӡ�
(3)ʢװ�Ҵ��IJ���������________________________
����������װ�ý����Ҵ��Ĵ�����ʵ�飬��������������Cװ�õ��Թ���ʢ����ˮ�Ҵ���(�̶��ͼг�װ������ȥ)
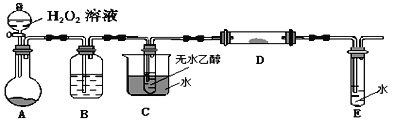
(4)װ��AԲ����ƿ�ڵĹ���������_____��B�е��Լ���_____C��������______��
(5)��ʵ������Ҫ���ȵ�װ����_________________ (��װ���µ���ĸ)��
(6)д��D��������Ӧ�Ļ�ѧ����ʽ_________________________________��
(7)����E�е���������Ҫ���Լ�Ϊ______________________��
���𰸡�����Ӵ�������߷�Ӧ���� �ǻ� ��Һ©�� MnO2 Ũ���� Ԥ�ȷ�Ӧ������� (�ṩ�Ҵ�����) CD 2CH3CH2OH+O2![]() 2CH3CHO+2H2O ������Һ(������������ͭ����Һ)
2CH3CHO+2H2O ������Һ(������������ͭ����Һ)
��������
��(1)�Ʒ���������Ӵ��棬��߷�Ӧ���ʣ�
(2)������Ҵ������ʵ����������ƹ������Ҵ���ȫ��Ӧ���������������ʵ���������Ҵ����ܹ�����ȡ����ԭ������������ԭ����ȷ���Ҵ��Ľṹ��
(3)���������ṹȷ���Ҵ�ʢ�ŵ��������ƣ�
��A���ǹ��������ڶ������̴�����������ˮ��������B�����������е�ˮ������C�Ǽ����Ҵ��õ��Ҵ���������D��D�м���ʱ�Ҵ�������Ϊ��ȩ����E�����շ�Ӧ��������ȩ������ʵ��Ŀ��ȷ�����������ü�ʹ�÷�����
��(1)ʵ��������Ҫ����ɺ�С�Ŀ�����Ŀ������Ӵ��������߷�Ӧ���ʣ�
(2)�Ҵ�������Ϊ��m(C2H6O)=1.15g�����Ҵ������ʵ���Ϊ��n(C2H6O)=1.15g ��46g/mol=0.025mol���ռ����������ƽ��Ϊ0.28��(����ɱ�״̬)�������ʵ���Ϊn(H2)= 0.28L��22.4L/mol=0.0125mol Ҳ����Ϊ0.025molH���ɼ�1��C2H6O�����У�ֻ��1��H���Ա�Na�û�����˵��C2H6O�������6��H�У���1��������5���Dz�ͬ�ģ��Ҵ��Ľṹ��ʽΪCH3CH2OH�����Ʋ�H2�����Ҵ��������ǻ�Hԭ�ӣ�
(3)����װ��ͼ��֪ʢװ�Ҵ��IJ��������Ƿ�Һ©����
��(4)A����H2O2��MnO2������������ˮ����������Ӧ�Ļ�ѧ����ʽΪ2H2O2 ![]() 2H2O+O2����B�������������������е�ˮ����������Ũ��������ˮ����C�������Ǽ����Ҵ��õ��Ҵ���������D������C������ΪԤ�ȷ�Ӧ������壻
2H2O+O2����B�������������������е�ˮ����������Ũ��������ˮ����C�������Ǽ����Ҵ��õ��Ҵ���������D������C������ΪԤ�ȷ�Ӧ������壻
(5)�ڱ�ʵ���У�C����Ȳ����Ҵ�������E����ȣ�ʹ�Ҵ�������O2��Cu���·���������Ӧ������ȩ������Ҫ���ȵ�װ����CD��
(6)��Dװ�����Ҵ�������������ȩ����Ӧ�ķ���ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
(7)�Ҵ�������Ϊ��ȩ����ȩ����ȩ�������л�ԭ�ԣ��ܱ�������Һ������������ͭ����Һ�����������֤�Ҵ�����������ȩ�Ļ�ѧ�Լ�Ϊ������Һ������������ͭ����Һ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����л����˵���У���ȷ��һ����(����)
�ٵ��ۡ���֬��һ�������¶��ܷ���ˮ�ⷴӦ�� �ڵ��ۺ���ά�ػ�Ϊͬ���칹�塡
��ʳ�����������࣬ʯ�����������ࡡ ��ʯ�͵ķ����ú�������������˻�ѧ�仯
�ݵ�������Ʊ���ɫ���ڼ�����������������������Cu(OH)2����Һ������Ӧ
A. �٢ڢ�B. �٢ڢ�C. �٢ۢ�D. �ۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Bilirubin��һ�������������·����ֽⷴӦ����Ӧ��Ũ���淴Ӧʱ��仯��ͼ��ʾ�����㷴Ӧ48minƽ����Ӧ���ʺ��ƲⷴӦ16minʱ��Ӧ���Ũ�ȣ����Ӧ��( )
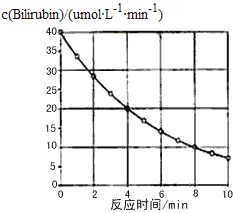
A. 2.5��mol/(L��min)�� 2.0��molB. 2.5��mol/(L��min)�� 2.5��mol
C. 3.0��mol/(L��min)�� 3.0��molD. 5.0��mol/(L��min)�� 3.0��mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��ӦA+B=C+D�������仯��ͼ��ʾ(E1��E2��Ϊ��ֵ)������˵����ȷ����

A. �ƻ���Ӧ���еĻ�ѧ�������յ�����С���γ��������л�ѧ�����ų�������
B. �÷�Ӧ���յ�����Ϊ(E1-E2)
C. A��B��������һ������C��D��������
D. �÷�Ӧֻ���ڼ��������²��ܽ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�����ȼ�ϵ�ص�˵����ȷ����( )
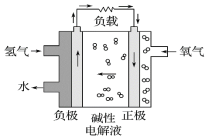
A. ��װ���ɵ���ת��Ϊ��ѧ��
B. �����ĵ缫��ӦʽΪO2+2H2O+4e-=4OH-
C. ����22.4L O2ת��4mol����
D. �ŵ�����м��Ե��Һ��OH-�����ʵ���Ũ�Ȳ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�ʵ��װ��(�гֺ�β������װ����ʡ��)���е���Ӧʵ�飬���ܴﵽʵ��Ŀ�ĵ���

A. ���ü�װ�ã���֤SO2��H2SO3����������
B. ������װ�ã���֤Ԫ�صķǽ����ԣ�C1>C>Si
C. ���ñ�װ�ã�����NH3�ĸ���ռ���β������
D. ���ö�װ�ã���֤ŨH2SO4������ˮ�ԡ�ǿ�����ԣ�SO2����Ư���ԡ���ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���̷�(FeSO4��7H2O)�ڹ�ҵ�Ͽ������������Ρ��������켰����ȡ���������������м(����������������������)Ϊԭ�����������̷���һ�ַ�����

25�� | pHֵ |
����H2S��Һ | 3.9 |
SnS������ȫ | 1.6 |
FeS��ʼ���� | 3.0 |
FeS��ʼ���� | 5.5 |
��1�������Ƶõ��̷��������Ƿ���Fe3�������ѡ�õ��Լ�Ϊ________��
A��KSCN��Һ B��NaOH��Һ C��KMnO4��Һ D��������Һ
��2���������У�ͨ�����������͵�Ŀ����______________________________________������Һ���������ữ��pH��2��Ŀ����__________________________________��
��3����������˳������Ϊ_________________����ȴ�ᾧ��____________________��
��4���������õ����̷�������������ˮϴ�ӣ���Ŀ���ǣ��ٳ�ȥ������渽�ŵ���������ʣ���_________________________________________________________��
��5���ⶨ�̷���Ʒ��Fe2�������ķ����ǣ�a.��ȡ2.850 g�̷���Ʒ���ܽ⣬��250 mL����ƿ�ж��ݣ�b.��ȡ25.00 mL������Һ����ƿ�У�c.�������ữ��0.010 00 mol��L��1 KMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00 mL��
�ٵζ�ʱʢ��KMnO4��Һ������Ϊ________________________(����������)��
�ڼ���������Ʒ��FeSO4��7H2O����������Ϊ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Դ��������������ͷ�չ����Ҫ���ʻ�����������Դ�ĺ������ú�����Դ�ĺ��������ǵ���������ٵ��Ͼ�����,�ش���������:
��1���ҹ���������������úΪ��Ҫȼ�ϵĹ���,���й���ú��ȼ�ϵ��۵���ȷ���� ___________________(����ĸ)��
A��ú����Ҫ�Ļ���ԭ�ϣ���ú��ȼ�ϼ�ȼ�յ�̫��ϧ,Ӧ���ۺ�����
B��ú�Ƿ������ܸߵĹ���ȼ�ϣ��ҹ�ú̿��Դ��Լ��У����ɳɱ��ͣ���ú��ȼ��ʵ��
C��úȼ��ʱ������������������̳����Ի�����Ⱦ����
D��ͨ���ྻú��������ú��������Һ�����Լ�����������������ȼú��Ⱦ���������úȼ�յ���������
��2���Ҵ���δ����ȼ������ѡ������Һ��ȼ�ϡ�2.0 g�Ҵ���ȫȼ������Һ̬ˮ�ų�59.43 kJ�����������Ҵ�ȼ�յ��Ȼ�ѧ����ʽΪ____________________________________________________________��
��3������C3H8(g)��C3H6(g)+H2(g) ��H=+b kJmol1(b��0)�ķ�Ӧ�У���Ӧ����е�������________(����������������������С����)��������е�����������ô�ڻ�ѧ��Ӧʱ����Ӧ�����Ҫ________(�����ų�������������)��������ת��Ϊ�����
��4��������ˮ��ȡ������Դ�����������о�������ȷ����________________
A�����ˮ����������ǿ���ȼ�յ����ʣ���˿��о���ˮ���ֽ������£�ʹ���Ϊ������Դ
B���跨��̫����۽����������£�ʹˮ�ֽ��������
C��Ѱ�Ҹ�Ч������ʹˮ�ֽ����������ͬʱ�ͷ�����
D��Ѱ��������������ڿ���������Դ���Էֽ�ˮ��ȡ����
��5����֪���������Ȼ�ѧ����ʽ��
A��2H2(g)+O2(g) ===2H2O(l) ��H��-571.6 kJmol-1 B��C3H8(g)+5O2(g) ===3CO2(g)+4 H2O(l) ��H��-2 220 kJmol-1�����У��ܱ�ʾȼ���ȵ��Ȼ�ѧ����ʽΪ___________��A��B������ȼ����Ϊ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[��ѧ-��ѡ��3�����ʽṹ������]������������־����־�����������й��ڰ�ͭ�ļ��أ���������ͭ��ͭ���Ͻ��������⣬����Ҫ������ң������������������Ʒ���ش��������⣺
��1����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ_________��3d�ܼ��ϵ�δ�ɶԵĵ�����Ϊ______��
��2�����������ڰ�ˮ�γ�[Ni(NH3)6]SO4��ɫ��Һ��
��[Ni(NH3)6]SO4�������ӵ����幹����_____��
����[Ni(NH3)6]2+��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ______���ṩ�µ��ӶԵijɼ�ԭ����_____��
�����ķе㣨����������������������좣�PH3����ԭ����______������_____���ӣ����������������Ǽ�������������ԭ�ӵĹ���ӻ�����Ϊ_______��
��3������ͭ����������______���γɵľ��壺Ԫ��ͭ�����ĵڶ������ֱܷ�Ϊ��ICu=1959kJ/mol��INi=1753kJ/mol��ICu>INi��ԭ����______��
��4��ij����ͭ�Ͻ�����������ṹ��ͼ��ʾ��

��������ͭԭ������ԭ�ӵ�������Ϊ_____��
�����Ͻ���ܶ�Ϊdg/cm3����������a=________nm��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com