
��ҵ������Ҫ����NaCl��NaHCO3���ʣ�Ϊ�˼�������ĺ������������ζ��ⶨ�ܼ�ȣ���Ӧ����ΪNaCl��CO2��H2O����������һ����ҵ����ǡ����ȫ��Ӧʱ����ҺpH 3.8��3.9(�ζ�ʱNaHCO3Ҳ���к�)����ҵ������ܼ�Ȳⶨֵͨ����Na2CO3%��Na2O%��ʾ��
��Ҫʵ�鲽�裺��ȷ��ȡ��ҵ��������2.00 g����������ˮ���Ƴ����һ������Һ����ȷ��ȡһ������������Һ������0.01 mol��L��1�����Һ�ζ��������ܼ�ȡ�
��ش��������⣺
(1)��ȡ���幤ҵ��������ʱ��Ϊʲô����ȡ������Щ����������ȣ�
________________________________________________________________________��
(2)��ȷ��ȡ����������Ϊ��������ʵ�飬��ѡ���ձ�������������ʽ�ζ��ܡ���ʽ�ζ��ܡ���ƿ֮�⡣����Ҫ��������Ʒ(�Լ�����)��
____________________________________________________________��
(3)�ڵζ������У�Ӧѡ�õ�ָʾ����
________________________________________________________________________��
(4)��������ȡ�Ĵ��������У�������2%��NaCl�⣬�����������ֻ��NaHCO3����ôNaHCO3�����Ĵ�С�Ը������ܼ�ȵIJⶨֵ�к�Ӱ�죿
________________________________________________________________________
________________________________________________________________________��
(1)������Ʒ���ʷֲ���һ�����ȣ����ȡ��ʱ�����ѹ����Ͼ��ȣ���ȡ����һЩʱ�����С��ʹ���������ȷ
(2)����ƿ����ͷ�ιܡ�����̨���ζ��ܼ�
(3)����
(4)�������к�NaHCO3�������٣��������ܼ�ȵIJⶨֵ����Խϴ���������NaHCO3�ĺ����϶࣬���������ܼ�ȵIJⶨֵ��С
��������
������������������к͵ζ�ԭ�����ⶨ��ҵ������ܼ�ȣ�����ӦŪ�������Ϣ��pHΪ3.8��3.9ʱ����������ʲô������ʣ��NaHCO3������NaCl��CO2��ˮ���ݴ˿�ѡ����ʵ�ָʾ����(3)��Ϊ��Ӧ���������У���һ����ӦΪNa2CO3��HCl===NaHCO3��NaCl(�������̪�����ڴ�ʱ��ɫ��ȥ��������NaHCO3�����нϴ����)���ڶ�����ӦΪNaHCO3��HCl===NaCl��CO2����H2O����������CO2����Һ��pH��3.8��3.9֮�䣬�����ɻ�ɫ���ɫpHΪ4.4���ȽϽӽ�����ѡ���ȽϺá�
���㣺����ʵ�����������������ָʾ����ѡ���Լ�ʵ��������
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ��������������ѵ��������������ѧ���淶�Ͻ���ʵ����ơ���������������������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ����������������������ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ��еĻ��ṩһЩ�µ���Ϣ�����Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ�


 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
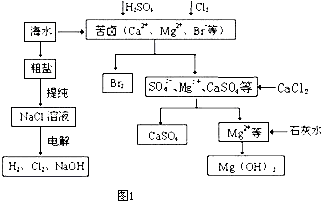
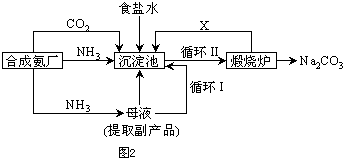
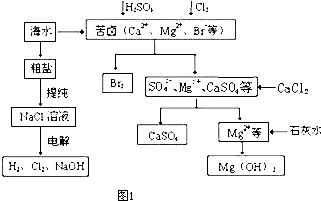
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ������

 NaCl(l)+ K(g)����ҵ��Ӧ������Щ��ʩ����ʹ��Ӧ������Ӧ�����ƶ���___________________
NaCl(l)+ K(g)����ҵ��Ӧ������Щ��ʩ����ʹ��Ӧ������Ӧ�����ƶ���___________________ �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������е潭ʵ����ѧ�߶����ϣ����л�ѧ�Ծ��������棩 ���ͣ������


 CO2+H2
CO2+H2�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com