
MnO2�Ǽ��̵�ز���������ͨ����������֮һ���ڻ��Բ���MnO2�м���CoTiO3������, ��������������ʣ��Ż����̵�ص����ܡ�
��1��д����̬Mnԭ�ӵĺ�������Ų�ʽ ��
��2��CoTiO3����ṹģ����ͼ����CoTiO3������1��Ti ԭ�ӡ�1��Co ԭ����Χ���������O��Ŀ�ֱ�Ϊ ���� ����
��3���������ѣ�TiO2���dz��õġ����нϸߴ����Ժ��ȶ��ԵĹ��������������ˮ������O2����������£��ɽ�CN��������CNO���������õ�N2����CNO����Ϊ�ȵ�����ķ��ӡ����ӻ�ѧʽ�ֱ�Ϊ �� ����дһ�֣���
 ��4�������谷��һ�ֺ����������ṹ��ʽ����ͼ�������谷�����е�ԭ�ӹ���ӻ������� ��1 mol�����谷�����ЦҼ�����ĿΪ ��
��4�������谷��һ�ֺ����������ṹ��ʽ����ͼ�������谷�����е�ԭ�ӹ���ӻ������� ��1 mol�����谷�����ЦҼ�����ĿΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ⶨ��C2H4O��C6H6��ɵĻ������������������Ϊ8%����λ������̼����������Ϊ
A��8.5% B��78% C��14% D��84%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ɫ��ѧ�����У�����״̬�Ƿ�Ӧ����ԭ��ȫ��ת��Ϊ���ƵõIJ����ԭ��������Ϊ100%������CH3C��CH�ϳ�CH2===C(CH3)COOCH3�Ĺ����У���ʹԭ�������ʴﵽ��ߣ�����Ҫ�����ķ�Ӧ����(���� )
A��CO2��H2O B��CO��CH3OH
C��CH3OH��H2 D��H2��CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ԫ��X��Y��Z������X��Yλ��ͬһ���壬Y��Zλ��ͬһ���ڡ�Xԭ�ӵ�����������������Ӳ�����3����Zԭ�ӵĺ����������Yԭ����1�����бȽ���ȷ���ǣ� ��
A. ��̬�⻯����ȶ��ԣ�Z < Y < X B.���������ˮ�������ԣ�Z > Y
C. ԭ�Ӱ뾶��Z < Y < X D. Ԫ�طǽ����ԣ�Z> Y > X
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������һ�ֽྻ������������Դ����������(��Ҫ�ɷ�ΪCO��CO2��H2��)��H2��ϣ����ϳɼ״��������������õķ���֮һ��
(1)������Ӧ�Ĵ�������Cu��Zn��Al��Ԫ�ء�д����̬Znԭ�ӵĺ�������Ų�ʽ___________________________________________________________��
(2)���ݵȵ���ԭ����д��CO���ӵĽṹʽ_________________________��
(3)�״��������ɵõ���ȩ����ȩ������Cu(OH)2�ļ�����Һ��Ӧ����Cu2O������
�ټ״��ķе�ȼ�ȩ�ĸߣ�����Ҫԭ����________________________________��
��ȩ������̼ԭ�ӹ�����ӻ�����Ϊ___________________��
�ڼ�ȩ���ӵĿռ乹����__________________��1 mol��ȩ�����ЦҼ�����ĿΪ____________��
����1��Cu2O������(�ṹ��ͼ��ʾ)����������Cu+��ĿΪ__________��
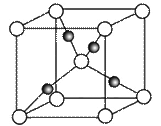
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йػ�ѧ������ȷ���� �� ��
����ϩ�����ʽC2H4 ���Ҵ��Ľṹ��ʽC2H6O
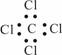 |
�����Ȼ�̼�ĵ���ʽ ����Ȳ�Ľṹ��ʽCHCH
������ĽṹʽCH3CH3 ����ȩ�Ľṹ��ʽCH3COH
A��ȫ�� B��ȫ�� C���ۢܢ� D���ۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ���� �� ��
A��ʵ�����ô���ʯ����ᷴӦ��ȡ������̼��
CaCO3+2H+=Ca2��+CO2��+H2O
B��������������������Һ����: CH2ClCOOH��OH- ��CH2ClCOO-��H2O
C������Ũ���ᡢŨ����Ļ��Һ��������������
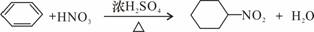
D����������Һ������ȩ�е�ȩ����
CH3CHO +2[Ag(NH3)2]++2OH�� CH3COO��+NH4+ +3NH3+2Ag��+ H2O
CH3COO��+NH4+ +3NH3+2Ag��+ H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ���� �� ��
A������������������
B�����������ڶ��ǣ��ܷ���ˮ��
C����֬���ͺ�֬��֮�֣�����������
D�������ʡ���֬���������������Ӫ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com