��X��Y��Z����Ԫ�أ���Z��Y����ɻ�����ZY
3��ZY
3��Һ�����ӳ���ɫ��֤��ZY
3ΪFeCl
3������X
2-��Y
-����Y����̬�⻯����Ӿ�����ͬ�ĵ�����Ϊ18��������XΪSԪ�أ��ƶ�XYZԪ�طֱ�Ϊ��S��Cl��Fe��
��1����X��ZԪ���γ�ZX
2�������ѧʽΪ��FeS
2����FeS
2�ĵ���ʽΪ��
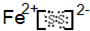
�����к��еĻ�ѧ�������Ӽ����ۼ����ʴ�Ϊ��
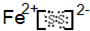
�����ۼ� ���Ӽ���
��2����ZY
3��FeCl
3����Һ�����ˮ�ɵõ����ɫҺ��Ϊ�����������壬��Ӧ�����ӷ���ʽ�ǣ�Fe
3++3H
2O
Fe��OH��
3+3H
+���ʴ�Ϊ��Fe
3++3H
2O
Fe��OH��
3+3H
+��
a������ͨ����Һ��ʱ�γɹ����ġ�ͨ·���ǽ�������ʣ������˶��������a���ϣ�
b������缫ֱͨ�������һ������Һ����ɫ����ǽ���ĵ�Ӿ���ʣ�������������������ӣ�ͨ��ʱ����ij�缫��������������������������ɵ����ӣ�ͨ��������������b���ϣ�
c�����Һ���м�����������Һ������ְ�ɫ������������c�����ϣ�
d������Һ����Ⱦ۳����ɳ��������ɡ����Ⱥ����������ֽ������������������������ɣ���d���ϣ�
�ʴ�Ϊ��abd
��3��X����ΪS�ڿ�����ȼ������һ����ɫ�д̼�����ζ����������ΪSO
2��
����֪һ�������£�ÿ1mol�����屻O
2��ȫ��������98.0kJ�����ݻ�ѧ��Ӧ����ʽд���÷�Ӧ���Ȼ�ѧ����ʽ��2SO
2��g��+O
2��g��=2 SO
3��g������H=-196kJ/mol����2molSO
2������1molO
2�ڴ������·�����Ӧ���ﵽƽ��ʱ�ų���������176.4kJ����Ϊ��Ӧ�ǿ��淴Ӧ��ʵ�ʷ�Ӧ�Ķ��������������ʵ���=
=1.8mol��Ϊ��������ת����=
��100%=90%���ʴ�Ϊ��2SO
2��g��+O
2��g��=2 SO
3��g������H=-196kJ/mol��90%��
��ԭ��ɫ�д̼�����ζ������SO
2�뺬1.5mol Y��һ�ֺ����ᣬ�����ij�γ�����ʵ������ȡ�������ж�ΪKClO
3����������ҺΪHClO
3����Һ�Ͷ�����������һ�������·�Ӧ��������һ��ǿ���һ�����������1.5��6.02��10
23������ת��ʱ�����ʵ���Ϊ1.5mol�����ݵ����غ��֪������������Ԫ�ػ��ϼ۴�+5�۱仯Ϊx��
SO
2��H
2SO
4��2e-
1 2
0.75mol 1.5mol
HClO
3�����ȵ��������5-x��e-
1 5-x
1.5mol 1.5mol
�õ�x=4
���������ﻯѧʽΪClO
2��
���ԵĶ���������������ʵ���֮��Ϊ0.75��1.5=1��2��
��Ӧ�Ļ�ѧ����ʽ�ǣ�SO
2+2HClO
3=H
2SO
4+2ClO
2��
�ʴ�Ϊ��SO
2+2HClO
3=H
2SO
4+2ClO
2��
��4��YΪClԪ�ص�����������Ӧˮ����Ļ�ѧʽ�ǣ�HClO
4��
�ٳ����£���20mL0.1mol?L
-1��HClO
4��ˮ��Һ��VmL0.1mol?L
-1�İ�ˮ����ǡ�÷�Ӧ��Ҫ��ˮ20ml������NH
4ClO
4��Һ������ǿ����ˮ�������ԣ�����Ϻ�pH=7��˵����ˮ��������20ml��
�ڳ����£���pH=2��HClO
4��ˮ��ҺV
1mL��V
2mL0.01mol?L
-1�İ�ˮ��Ϻ���Һ�����ԣ���V
1��V
2�Ĺ�ϵ����V
1��V
2ʱ��Һһ�������ԣ���V
1��V
2��Ҳ���ܳ����ԣ����Բ���ȷ������ѡD��
��ʱ��Һ�д�������Ũ�ȴ�С˳�����Ϊ��ǡ�÷�Ӧʱ��Һ������Ũ�ȴ�СΪ��c��ClO
4-����c��NH
4+����c��H
+����c��OH
-�����������Һ������Ũ�ȴ�СΪ��c��ClO
4-����c��H
+����c��NH
4+����c��OH
-���� ��ˮ�Թ�����Һ������Ũ�ȴ�СΪ��c��ClO
4-����c��NH
4+��=c��H
+����c��OH
-��
�ʴ�Ϊ��HClO
4 ������D��c��ClO
4-����c��NH
4+����c��H
+����c��OH
-����c��ClO
4-����c��H
+����c��NH
4+����c��OH
-�� �� c��ClO
4-����c��NH
4+��=c��H
+����c��OH
-��
��5��ZΪFe��Ԫ�صĸ���������Һ�еμ�����HI��Һ��Ӧ���������Ӻ���Һ�����������������Һ�ж����������Զ����������ӣ����������ӱ���ԭΪ���������ӣ����ᱵ��ԭΪһ�������������ӱ�����Ϊ���ʵ⣬����ԭ���غ�͵����غ���ƽд������Ӧ�����ӷ���ʽ��Fe
3++3NO
3-+12H
++10I
-=Fe
2++5I
2+3NO��+6H
2O��
�ʴ�Ϊ��Fe
3++3NO
3-+12H
++10I
-=Fe
2++5I
2+3NO��+6H
2O��



 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�



