
��֪��pHΪ4��5�Ļ����У�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⡣ijѧ�����õ�ⴿ��CuSO4��Һ�ķ����������ݵ缫������ͭ�������Լ��缫�ϲ��������������ⶨͭ�����ԭ����������ʵ���������£�
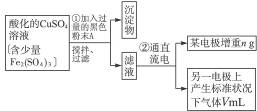
ͼ4-16
�Իش��������⣺
(1)������������A�Ļ�ѧʽΪ________________������A��������___________________,
(2)����������ò���������ͼ4-16��ʾ����AӦ��ֱ����Դ��__________����
(3)��ʼ��һ��ʱ�䣬��U�ι��ڿɹ۲쵽��������____________�������ܷ�Ӧ�����ӷ���ʽΪ________________________��
(4)����ʵ���������Ҫ����__________(����ĸ����ͬ)��������Ҫ����________________��
A.�������ǰ�缫������
B.����缫�ں�ɡ�����ǰ������������ˮ��ϴ
C.���µ���缫�ϵ�ͭ������ϴ������
D.�缫�ں�ɳ����IJ������밴��ɡ��������ٺ�ɡ��ٳ�����������
E.���п������ڵ�����£��缫��ɱ�����õ��º�ɷ�
(5)ͭ�����ԭ������Ϊ______________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| 11200n |
| V |
| 11200n |
| V |
�鿴�𰸺ͽ���>>
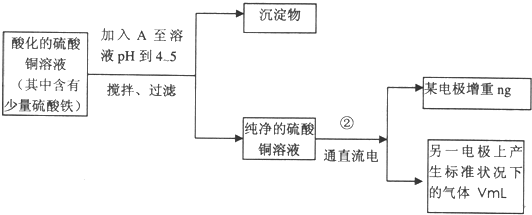
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
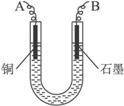
ͼ4-16
�Իش��������⣺
(1)������������A�Ļ�ѧʽΪ________________������A��������___________________,
(2)����������ò���������ͼ4-16��ʾ����AӦ��ֱ����Դ��__________����
(3)��ʼ��һ��ʱ�䣬��U�ι��ڿɹ۲쵽��������____________�������ܷ�Ӧ�����ӷ���ʽΪ________________________��
(4)����ʵ���������Ҫ����__________(����ĸ����ͬ)��������Ҫ����________________��
A.�������ǰ�缫������
B.����缫�ں�ɡ�����ǰ������������ˮ��ϴ
C.���µ���缫�ϵ�ͭ������ϴ������
D.�缫�ں�ɳ����IJ������밴��ɡ��������ٺ�ɡ��ٳ�����������
E.���п������ڵ�����£��缫��ɱ�����õ��º�ɷ�
(5)ͭ�����ԭ������Ϊ______________________��
�鿴�𰸺ͽ���>>
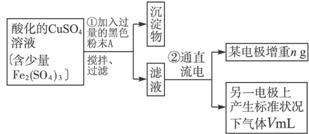
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
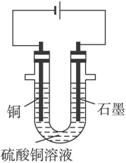
��1����������Fe2��SO4��3��ϡH2SO4��CuSO4��Һ�У������Թ����ĺ�ɫ��ĩA���衢���ˣ��õ��ϴ�����CuSO4��Һ��A�Ļ�ѧʽΪ______________������A��������__________________________��
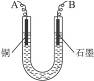
��2�����ϴ�����CuSO4��Һ������ͼ��ʾ��װ���н��е�⣬ʯī�缫�ϵĵ缫��ӦʽΪ_____________����ⷴӦ�����ӷ���ʽΪ__________________________��
��3��ʵ����ɺ�ʯī�缫������״���µ�����V mL��ͭ�缫����a g����Cu�����ԭ���������ô���a��V�ļ���ʽ��ʾ��Ϊ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ɽ��ʡΫ�����ٹ��и������ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com