
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪��25�桢1.013��105Pa�£�1mol������ȫȼ������Һ̬ˮ�ų�285kJ����������ش��������⣺
��֪��25�桢1.013��105Pa�£�1mol������ȫȼ������Һ̬ˮ�ų�285kJ����������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

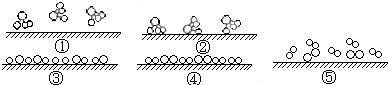
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡΫ���������и�����ѧ���������Ͽ��Ի�ѧ�Ծ� ���ͣ������
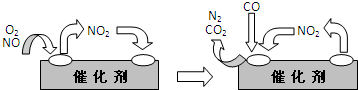
��10�֣��о�NOx��SO2��CO�ȴ�����Ⱦ����Ĵ�������������Ҫ���塣
��1��������CO��SO2�̵�����Ⱦ��һ�ַ����ǽ����ڴ���������ת��Ϊ����S��
��֪����CO��g��+ 1/2O2(g)��CO2��g�� ��H=��283.0KJ��mol-1
��S��s��+ O2(g)��SO2��g�� ��H=��296.0KJ��mol-1
�˷�Ӧ���Ȼ�ѧ����ʽ�� ��
��2��������������ɹ⻯ѧ�����ͳ�������ĵ���Ҫ���塣��֪��
�� CO��g��+NO2(g)��NO(g)+CO2(g) ��H=��aKJ��mol-1 (a��0)
�� 2CO��g��+2NO (g)��N2(g)+2CO2(g) ��H=��bKJ��mol-1 (b��0)
���ñ�״���� 3.36LCO��ԭNO2��N2��CO��ȫ��Ӧ��������������ת�Ƶ��ӵ����ʵ���Ϊ mol���ų�������Ϊ KJ���ú���a��b�Ĵ���ʽ��ʾ����
��3����CH4�� ����ԭNOxҲ�������������������Ⱦ�����磺
����ԭNOxҲ�������������������Ⱦ�����磺
��CH4��g��+4NO2(g)=4NO(g)+CO2(g)+2H2O(g) ��H1����574KJ��mol-1 ��
��CH4��g��+4NO (g)=2N2(g)+CO2(g)+2H2O(g) ��H2������
��1molCH4��ԭNO2��N2���������зų�������Ϊ867KJ�����H2= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ�Ƹ���Ӣɽһ�и�һ���£����л�ѧ�Ծ��������棩 ���ͣ������
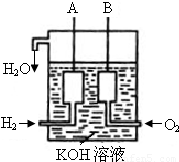
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com